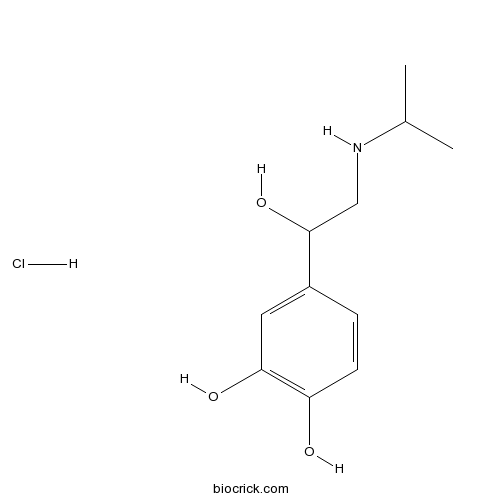
Isoprenaline HClβ-adrenergic receptor agonist CAS# 51-30-9 |
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- A-443654
Catalog No.:BCC1321
CAS No.:552325-16-3
- AKT inhibitor VIII
Catalog No.:BCC1334
CAS No.:612847-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 51-30-9 | SDF | Download SDF |
| PubChem ID | 5807 | Appearance | Powder |
| Formula | C11H18ClNO3 | M.Wt | 247.72 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Isoprenaline | ||
| Solubility | DMSO : 80 mg/mL (322.95 mM; Need ultrasonic) H2O : ≥ 50 mg/mL (201.84 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[1-hydroxy-2-(propan-2-ylamino)ethyl]benzene-1,2-diol;hydrochloride | ||
| SMILES | CC(C)NCC(C1=CC(=C(C=C1)O)O)O.Cl | ||
| Standard InChIKey | IROWCYIEJAOFOW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H17NO3.ClH/c1-7(2)12-6-11(15)8-3-4-9(13)10(14)5-8;/h3-5,7,11-15H,6H2,1-2H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard selective β-adrenoceptor agonist; vasorelaxant and bronchodilator. Activation of β2 receptors activates downstream PKA and ERK, and may stimulate NO-mediated endothelium-dependent smooth muscle relaxation. Active in vivo. Also available as part of the β-Adrenoceptor Agonist and Mixed Adrenergic. |

Isoprenaline HCl Dilution Calculator

Isoprenaline HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0368 mL | 20.1841 mL | 40.3682 mL | 80.7363 mL | 100.9204 mL |
| 5 mM | 0.8074 mL | 4.0368 mL | 8.0736 mL | 16.1473 mL | 20.1841 mL |
| 10 mM | 0.4037 mL | 2.0184 mL | 4.0368 mL | 8.0736 mL | 10.092 mL |
| 50 mM | 0.0807 mL | 0.4037 mL | 0.8074 mL | 1.6147 mL | 2.0184 mL |
| 100 mM | 0.0404 mL | 0.2018 mL | 0.4037 mL | 0.8074 mL | 1.0092 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Isoprenaline HCl is an agonist of β-adrenergic receptor [1].
The adrenergic receptors are a class of G protein-coupled receptors that are targets of norepinephrine and epinephrine. When reacting with epinephrine, β-adrenergic receptor causes vasodilation.
In human umbilical vein endothelial cells (HUVECs), isoprenaline (100 nM) significantly increased the expression of connexins Cx43 and also increased Cx40 and Cx37. Also, isoprenaline increased the number of coupling cells. Isoprenaline enhanced the formation of branches and complex tube networks [2].
In the isolated field stimulated rat vas deferens, isoprenaline inhibited contractions with EC50 value of 45.6 nM. In tissues in which β 2-adrenoreceptors were maximally blocked by timolol, isoprenaline inhibited contractility with EC50 value of 1.5 μM [1]. In CFW mice, IPR caused the maximal proliferative response of bone marrow cells and increased erythropoietic activity. These results suggested that isoprenaline activated beta-adrenergic receptor, which then increased the proliferative and differentiation activities of hemopoietic stem cells [3].
References:
[1]. Lotti VJ, Cerino D, Kling P. Characterization of the adrenoreceptor activities of isoprenaline in the field stimulated rat vas deferens: selective supersensitivity to beta 2-mediated responses following reserpine treatment. J Auton Pharmacol, 1982, 2(3): 169-174.
[2]. Dhein S, Gaertner C, Georgieff C, et al. Effects of isoprenaline on endothelial connexins and angiogenesis in a human endothelial cell culture system. Naunyn Schmiedebergs Arch Pharmacol, 2015, 388(1): 101-108.
[3]. Lipski S. Effect of beta-adrenergic stimulation by isoprenaline on proliferation and differentation of mouse bone marrow cells in vivo. Pol J Pharmacol Pharm, 1980, 32(3): 281-287.
- Tiratricol
Catalog No.:BCC4738
CAS No.:51-24-1
- Fluorouracil (Adrucil)
Catalog No.:BCC2135
CAS No.:51-21-8
- Benzimidazole
Catalog No.:BCC8847
CAS No.:51-17-2
- Procaine HCl
Catalog No.:BCC5072
CAS No.:51-05-8
- Pronethalol hydrochloride
Catalog No.:BCC5678
CAS No.:51-02-5
- 7ACC1
Catalog No.:BCC5553
CAS No.:50995-74-9
- 16-Methoxystrychnidin-10-One
Catalog No.:BCN8472
CAS No.:5096-72-0
- N-Methylcoclaurine
Catalog No.:BCN7079
CAS No.:5096-70-8
- Canadine
Catalog No.:BCN5626
CAS No.:5096-57-1
- Crotanecine
Catalog No.:BCN1963
CAS No.:5096-50-4
- Anacrotine
Catalog No.:BCN2057
CAS No.:5096-49-1
- Carminomycin
Catalog No.:BCC6379
CAS No.:50935-04-1, 39472-31-6
- Scopolamine
Catalog No.:BCN5045
CAS No.:51-34-3
- H-Hyp-OH
Catalog No.:BCC3250
CAS No.:51-35-4
- Norepinephrine
Catalog No.:BCN2206
CAS No.:51-41-2
- Epinephrine Bitartrate
Catalog No.:BCC4348
CAS No.:51-42-3
- Adrenaline
Catalog No.:BCN2191
CAS No.:51-43-4
- Histamine
Catalog No.:BCN2188
CAS No.:51-45-6
- L-Thyroxine
Catalog No.:BCC4917
CAS No.:51-48-9
- Propylthiouracil
Catalog No.:BCC4931
CAS No.:51-52-5
- Atropine
Catalog No.:BCN5639
CAS No.:51-55-8
- Homatropine Bromide
Catalog No.:BCC4570
CAS No.:51-56-9
- D-Amphetamine sulfate
Catalog No.:BCC5942
CAS No.:51-63-8
- 4'-Methoxyacetanilide
Catalog No.:BCC8711
CAS No.:51-66-1
Nitric oxide (NO) primarily accounts for endothelium-dependent component of beta-adrenoceptor-activated smooth muscle relaxation of mouse aorta in response to isoprenaline.[Pubmed:12596888]
J Smooth Muscle Res. 2002 Oct;38(4-5):87-99.
Isoprenaline is known to produce vascular relaxation through activation of beta-adrenoceptors. In recent years, beta-adrenoceptor-activated vascular relaxation has been the focus of pharmacological study in terms of both the receptor subtypes and the intracellular signaling mechanisms which trigger smooth muscle mechanical functions. In addition, the possible contribution of the endothelium to beta-adrenoceptor-activated relaxation of vascular beds has provoked considerable discussion, with consensus still to be established. In the present study, we examined the effects of isoprenaline on isolated mouse aortic smooth muscles to determine whether the presence of the endothelium plays a substantial role in the relaxation it produces. A possible role for nitric oxide (NO) as a primary endothelium-derived factor released in response to isoprenaline was also elucidated pharmaco-mechanically. In isolated thoracic and abdominal aortae pre-contracted with phenylephrine (3 x 10(-7)-10(-6) M), isoprenaline elicited relaxation in a concentration-dependent fashion (10(-9)-10(-5) M). In endothelium-denuded preparations, isoprenaline-elicited relaxation was reduced to 40-50% of the response obtained in endothelium-intact preparations. In the preparations treated with N(G)-nitro-L-arginine methyl ester (L-NAME, 3 x 10(-4) M; an NO synthase inhibitor) or 1H-[1,2,4]-oxadiazolo[4,3-a]-quinoxalin-1-one (ODQ, 10(-5) M; a soluble guanylyl cyclase inhibitor), isoprenaline-elicited relaxation was attenuated almost to the same degree as the response in endothelium-denuded preparations. The degree of endothelium-dependency in isoprenaline-elicited relaxation was largely diminished when treated with propranolol (3 x 10(-6) M). The present findings indicate that isoprenaline substantially relaxes the mouse aorta with both endothelium-dependent and -independent mechanisms. The endothelium-dependent component seems to correspond to about 50% of the isoprenaline-elicited relaxation, and is almost entirely due to endothelium-derived NO. Activation of propranolol (3 x 10(-6) M)-inhibitable beta-adrenoceptors seems to be primarily responsible for the NO-mediated endothelium-dependent pathway in isoprenaline-elicited relaxant response of mouse aorta.
beta 2-adrenergic receptor activates extracellular signal-regulated kinases (ERKs) via the small G protein rap1 and the serine/threonine kinase B-Raf.[Pubmed:10840035]
J Biol Chem. 2000 Aug 18;275(33):25342-50.
G protein-coupled receptors can induce cellular proliferation by stimulating the mitogen-activated protein (MAP) kinase cascade. Heterotrimeric G proteins are composed of both alpha and betagamma subunits that can signal independently to diverse intracellular signaling pathways including those that activate MAP kinases. In this study, we examined the ability of isoproterenol, an agonist of the beta(2)-adrenergic receptor (beta(2)AR), to stimulate extracellular signal-regulated kinases (ERKs). Using HEK293 cells, which express endogenous beta(2)AR, we show that isoproterenol stimulates ERKs via beta(2)AR. This action of isoproterenol requires cAMP-dependent protein kinase and is insensitive to pertussis toxin, suggesting that Galpha(s) activation of cAMP-dependent protein kinase is required. Interestingly, beta(2)AR activates both the small G proteins Rap1 and Ras, but only Rap1 is capable of coupling to Raf isoforms. beta(2)AR inhibits the Ras-dependent activation of both Raf isoforms Raf-1 and B-Raf, whereas Rap1 activation by isoproterenol recruits and activates B-Raf. beta(2)AR activation of ERKs is not blocked by expression of RasN17, an interfering mutant of Ras, but is blocked by expression of either RapN17 or Rap1GAP1, both of which interfere with Rap1 signaling. We propose that isoproterenol can activate ERKs via Rap1 and B-Raf in these cells.
Characterization of the adrenoreceptor activities of isoprenaline in the field stimulated rat vas deferens: selective supersensitivity to beta 2-mediated responses following reserpine treatment.[Pubmed:6292228]
J Auton Pharmacol. 1982 Sep;2(3):169-74.
1 Low concentrations of isoprenaline (EC50 = 45.6 nM) inhibited contractions in the isolated field stimulated rat vas deferens. This inhibitory effect was markedly attenuated by the postjunctional beta 2-adrenoreceptor antagonist timolol, but not affected by the prejunctional alpha 2 or postjunctional alpha 1-adrenoreceptor antagonists rauwolscine and prazosin, respectively. 2 In vas deferens of rats previously treated with reserpine, the postjunctional beta 2-adrenoreceptor-mediated inhibitory response to isoprenaline was markedly potentiated. 3 High concentrations of isoprenaline (EC50 = 1.5 microM) also inhibited contractility in tissues in which postjunctional beta 2-adrenoreceptors were maximally blocked by high concentrations of timolol. This contractile inhibition produced by isoprenaline was abolished by rauwolscine but not significantly altered by prazosin or pretreatment of the rats with reserpine indicating stimulation of prejunctional alpha 2-adrenoreceptors. 4 Rauwolscine pretreatment unmasked an ability of isoprenaline (EC50 = 17.1 microM) to produce enhancement of field stimulation-induced contractions. This response was abolished by prazosin but was unaffected by timolol or reserpinization indicating an action upon postjunctional alpha 1-adrenoreceptors. 5 The data indicate isoprenaline activates adrenoreceptor mechanisms in the field stimulated rat vas deferens by a direct action not dependent upon endogenous catecholamines and with an order of activity of beta 2 much greater than alpha 2 greater than alpha 1. Pretreatment with reserpine produces rapid and selective development of supersensitivity to the postjunctional beta 2-mediated inhibitory response of isoprenaline in this preparation.


