MK-2206 dihydrochlorideAkt1/2/3 inhibitor CAS# 1032350-13-2 |
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- Perifosine
Catalog No.:BCC3673
CAS No.:157716-52-4
- AKT inhibitor VIII
Catalog No.:BCC1334
CAS No.:612847-09-3
- GSK690693
Catalog No.:BCC2483
CAS No.:937174-76-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1032350-13-2 | SDF | Download SDF |
| PubChem ID | 46930998 | Appearance | Powder |
| Formula | C25H23Cl2N5O | M.Wt | 480.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 20 mg/mL (41.63 mM; Need ultrasonic) H2O : 3.81 mg/mL (7.93 mM; Need ultrasonic and warming) | ||
| Chemical Name | 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2H-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3-one;dihydrochloride | ||
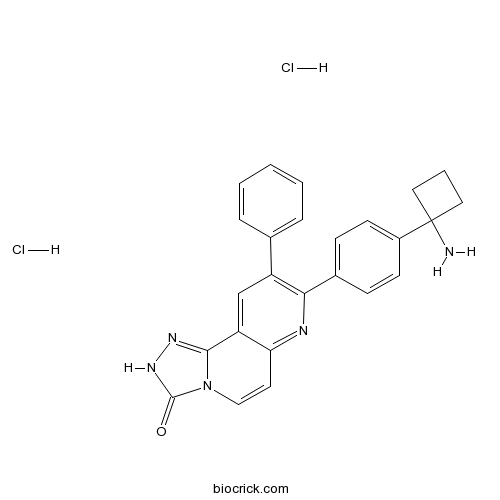
| SMILES | C1CC(C1)(C2=CC=C(C=C2)C3=C(C=C4C(=N3)C=CN5C4=NNC5=O)C6=CC=CC=C6)N.Cl.Cl | ||
| Standard InChIKey | HWUHTJIKQZZBRA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H21N5O.2ClH/c26-25(12-4-13-25)18-9-7-17(8-10-18)22-19(16-5-2-1-3-6-16)15-20-21(27-22)11-14-30-23(20)28-29-24(30)31;;/h1-3,5-11,14-15H,4,12-13,26H2,(H,29,31);2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MK-2206 2HCl is a highly selective inhibitor of Akt1/2/3 with IC50 of 8 nM/12 nM/65 nM, respectively. | |||||
| Targets | Akt1 | Akt2 | Akt3 | |||
| IC50 | 8 nM | 12 nM | 65 nM | |||
| Cell experiment:[1] | |
| Cell lines | Endometriotic stromal cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 100 nM, 2h |
| Applications | Inhibiting AKT with MK-2206 or MEK1/2 with U0126 for 24 hours in the absence of R5020 increased total and nuclear PRA and PRB protein levels in OSIS but not in eutopic endometrial stromal cells from disease-free patients from disease-free patients. MK-2206 and R5020 decreased OSIS viability and increased apoptosis. Trends toward decreased volumes of sc grafted endometriosis tissues were demonstrated with MK-2206 and progesterone. |
| Animal experiment:[1] | |
| Animal models | 5-week-old CD-1 nude mice |
| Dosage form | 360 mg/kg/d, 15 days, oral Gavage |
| Application | No significant interaction between MK-2206 and progesterone (P=0.628). Trends toward decreased tumor volume were noted with MK-2206 (P=0.077) and progesterone (P=0.087). Treatment with MK-2206 decreased levels of Ki67. Levels of cleaved caspase-3 (CC3) were very low in E and E +P-treated grafts, whereas MK-2206 increased CC3 levels, especially in the presence of P. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: 1. Eaton JL1, Unno K, Caraveo M et al. Increased AKT or MEK1/2 activity influences progesterone receptor levels and localization in endometriosis. J Clin Endocrinol Metab. 2013 Dec;98(12):E1871-9. | |

MK-2206 dihydrochloride Dilution Calculator

MK-2206 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0816 mL | 10.4082 mL | 20.8164 mL | 41.6328 mL | 52.041 mL |
| 5 mM | 0.4163 mL | 2.0816 mL | 4.1633 mL | 8.3266 mL | 10.4082 mL |
| 10 mM | 0.2082 mL | 1.0408 mL | 2.0816 mL | 4.1633 mL | 5.2041 mL |
| 50 mM | 0.0416 mL | 0.2082 mL | 0.4163 mL | 0.8327 mL | 1.0408 mL |
| 100 mM | 0.0208 mL | 0.1041 mL | 0.2082 mL | 0.4163 mL | 0.5204 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MK-2206 dihydrochloride is a selective inhibitor of Akt1/2/3. MK-2206 inhibites the phosphorylation of Thr308 and Ser 473 of Akt. MK-2206 suppresses Akt signalling pathway and promoting cancer cell death as a single agent as well as in combination with other chemotherapeutic agents. MK-2206 enhance the sensitivity to through apoptosis and enhance the sensitivity to rapamycin via reactive oxygen species. Combination of MK-2206 with etoposide or rapamycin significantly increase antitumor growth effect.
References
1. Pant A, Lee II, Lu Z, Rueda BR, Schink J, Kim JJ.Inhibition of AKT with the Orally Active Allosteric AKT Inhibitor, MK-2206, Sensitizes Endometrial Cancer Cells to Progestin.PLoS One. 2012;7(7):e41593. Epub 2012 Jul 24.
2. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. TA Yap, L Yan, A Patnaik, I Fearen. Journal of Clinical Oncology 2011
- D-Arabinose
Catalog No.:BCN3791
CAS No.:10323-20-3
- A939572
Catalog No.:BCC5305
CAS No.:1032229-33-6
- Fmoc-Cys(Trt)-OH
Catalog No.:BCC3479
CAS No.:103213-32-7
- Fmoc-Tyr(3,5-I2)-OH
Catalog No.:BCC3264
CAS No.:103213-31-6
- Pranlukast
Catalog No.:BCC4827
CAS No.:103177-37-3
- 14-Norpseurotin A
Catalog No.:BCN7262
CAS No.:1031727-34-0
- UNC 3230
Catalog No.:BCC5618
CAS No.:1031602-63-7
- 4-(4-(Dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-(hydroxymethyl)benzonitrile hydrobromide
Catalog No.:BCC8648
CAS No.:103146-26-5
- ABT-046
Catalog No.:BCC1326
CAS No.:1031336-60-3
- Kinetensin (human)
Catalog No.:BCC5845
CAS No.:103131-69-7
- 2-Amino-6-chloropurine
Catalog No.:BCC8540
CAS No.:10310-21-1
- Bakuchiol
Catalog No.:BCN5845
CAS No.:10309-37-2
- L-655,240
Catalog No.:BCC7156
CAS No.:103253-15-2
- BAY 80-6946 (Copanlisib)
Catalog No.:BCC4986
CAS No.:1032568-63-0
- GNE-477
Catalog No.:BCC8049
CAS No.:1032754-81-6
- GDC-0980 (RG7422)
Catalog No.:BCC4992
CAS No.:1032754-93-0
- PTIQ
Catalog No.:BCC7953
CAS No.:1032822-42-6
- GSK1292263
Catalog No.:BCC3786
CAS No.:1032823-75-8
- LDK378
Catalog No.:BCC3691
CAS No.:1032900-25-6
- Taltirelin
Catalog No.:BCC5271
CAS No.:103300-74-9
- Pre-schisanartanin B
Catalog No.:BCN5846
CAS No.:1033288-92-4
- 3-Oxo-4-aza-5-alpha-androstane-17β-carboxylic acid
Catalog No.:BCC8641
CAS No.:103335-55-3
- L-364,373
Catalog No.:BCC7445
CAS No.:103342-82-1
- 1-Methyl-L-4,5-dihydroorotic acid
Catalog No.:BCC8472
CAS No.:103365-69-1
Context-dependent antagonism between Akt inhibitors and topoisomerase poisons.[Pubmed:24569089]
Mol Pharmacol. 2014 May;85(5):723-34.
Signaling through the phosphatidylinositol-3 kinase (PI3K)/Akt pathway, which is aberrantly activated in >50% of carcinomas, inhibits apoptosis and contributes to drug resistance. Accordingly, several Akt inhibitors are currently undergoing preclinical or early clinical testing. To examine the effect of Akt inhibition on the activity of multiple widely used classes of antineoplastic agents, human cancer cell lines were treated with the Akt inhibitor A-443654 [(2S)-1-(1H-indol-3-yl)-3-[5-(3-methyl-2H-indazol-5-yl)pyridin-3-yl]oxypropan-2-a mine; ATP-competitive] or MK-2206 (8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2H-[1,2,4]triazolo[3,4-f][1,6]naphthyri din-3-one;dihydrochloride; allosteric inhibitor) or with small interfering RNA (siRNA) targeting phosphoinositide-dependent kinase 1 (PDK1) along with cisplatin, melphalan, camptothecin, or etoposide and assayed for colony formation. Surprisingly different results were observed when Akt inhibitors were combined with different drugs. Synergistic effects were observed in multiple cell lines independent of PI3K pathway status when A-443654 or MK-2206 was combined with the DNA cross-linking agents cisplatin or melphalan. In contrast, effects of the Akt inhibitors in combination with camptothecin or etoposide were more complicated. In HCT116 and DLD1 cells, which harbor activating PI3KCA mutations, A-443654 over a broad concentration range enhanced the effects of camptothecin or etoposide. In contrast, in cell lines lacking activating PI3KCA mutations, partial inhibition of Akt signaling synergized with camptothecin or etoposide, but higher A-443654 or MK-2206 concentrations (>80% inhibition of Akt signaling) or PDK1 siRNA antagonized the topoisomerase poisons by diminishing DNA synthesis, a process that contributes to effective DNA damage and killing by these agents. These results indicate that the effects of combining inhibitors of the PI3K/Akt pathway with certain classes of chemotherapeutic agents might be more complicated than previously recognized.
Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells.[Pubmed:21205925]
J Pharmacol Exp Ther. 2011 Apr;337(1):155-61.
Hepatocellular carcinoma (HCC) is one of the most common potentially lethal human malignancies worldwide. Sorafenib, a tyrosine kinase inhibitor, was recently approved by the United States Food and Drug Administration for HCC. In this study, we established two sorafenib-resistant HCC cell lines from Huh7, a human HCC cell line, by long-term exposure of cells to sorafenib. Sorafenib induced significant apoptosis in Huh7 cells; however, Huh7-R1 and Huh7-R2 showed significant resistance to sorafenib-induced apoptosis at the clinical relevant concentrations (up to 10 muM). Thorough comparisons of the molecular changes between Huh7 and resistant cells showed that the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway played a significant role in mediating acquired resistance to sorafenib in Huh7-R1 and Huh7-R2 cells. Phospho-Akt and p85 (a regulatory subunit of PI3K) were up-regulated, whereas tumor suppressor phosphatase and tensin homolog were down-regulated in these resistant cells. In addition, ectopic expression of constitutive Akt in Huh7 demonstrated similar resistance to sorafenib. The knockdown of Akt by RNA interference reversed resistance to sorafenib in Huh7-R1 cells, indicating the importance of Akt in drug sensitivity. Furthermore, the combination of 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-1,2,4-triazolo[3,4-f][1,6]naphthyridin-3 (2H)-one dihydrochloride (MK-2206), a novel allosteric Akt inhibitor, and sorafenib restored the sensitivity of resistant cells to sorafenib-induced apoptosis. In conclusion, activation of PI3K/Akt signaling pathway mediates acquired resistance to sorafenib in HCC, and the combination of sorafenib and MK-2206, an Akt inhibitor, overcomes the resistance at clinical achievable concentrations.


