L-364,373KV7.1 channel activator, activates IKs CAS# 103342-82-1 |
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- Balaglitazone
Catalog No.:BCC1395
CAS No.:199113-98-9
- Inolitazone
Catalog No.:BCC1652
CAS No.:223132-37-4
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- Aleglitazar
Catalog No.:BCC1337
CAS No.:475479-34-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103342-82-1 | SDF | Download SDF |
| PubChem ID | 656755 | Appearance | Powder |
| Formula | C25H20FN3O | M.Wt | 397.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | R-L3 | ||
| Solubility | Soluble to 100 mM in DMSO | ||
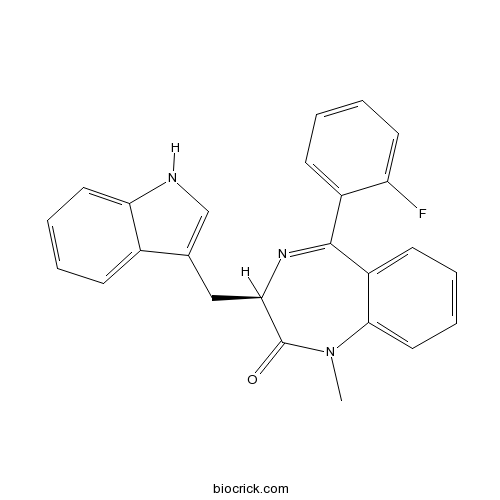
| Chemical Name | (3R)-5-(2-fluorophenyl)-3-(1H-indol-3-ylmethyl)-1-methyl-3H-1,4-benzodiazepin-2-one | ||
| SMILES | CN1C2=CC=CC=C2C(=NC(C1=O)CC3=CNC4=CC=CC=C43)C5=CC=CC=C5F | ||
| Standard InChIKey | CGBANSGENFERAT-JOCHJYFZSA-N | ||
| Standard InChI | InChI=1S/C25H20FN3O/c1-29-23-13-7-4-10-19(23)24(18-9-2-5-11-20(18)26)28-22(25(29)30)14-16-15-27-21-12-6-3-8-17(16)21/h2-13,15,22,27H,14H2,1H3/t22-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Activator of KV7.1 (KCNQ1) channels. Activates the cardiac IKs current that decreases action potential duration (APD) in cardiac myocytes. |

L-364,373 Dilution Calculator

L-364,373 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5161 mL | 12.5805 mL | 25.161 mL | 50.3221 mL | 62.9026 mL |
| 5 mM | 0.5032 mL | 2.5161 mL | 5.0322 mL | 10.0644 mL | 12.5805 mL |
| 10 mM | 0.2516 mL | 1.2581 mL | 2.5161 mL | 5.0322 mL | 6.2903 mL |
| 50 mM | 0.0503 mL | 0.2516 mL | 0.5032 mL | 1.0064 mL | 1.2581 mL |
| 100 mM | 0.0252 mL | 0.1258 mL | 0.2516 mL | 0.5032 mL | 0.629 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-Oxo-4-aza-5-alpha-androstane-17β-carboxylic acid
Catalog No.:BCC8641
CAS No.:103335-55-3
- Pre-schisanartanin B
Catalog No.:BCN5846
CAS No.:1033288-92-4
- Taltirelin
Catalog No.:BCC5271
CAS No.:103300-74-9
- LDK378
Catalog No.:BCC3691
CAS No.:1032900-25-6
- GSK1292263
Catalog No.:BCC3786
CAS No.:1032823-75-8
- PTIQ
Catalog No.:BCC7953
CAS No.:1032822-42-6
- GDC-0980 (RG7422)
Catalog No.:BCC4992
CAS No.:1032754-93-0
- GNE-477
Catalog No.:BCC8049
CAS No.:1032754-81-6
- BAY 80-6946 (Copanlisib)
Catalog No.:BCC4986
CAS No.:1032568-63-0
- L-655,240
Catalog No.:BCC7156
CAS No.:103253-15-2
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- D-Arabinose
Catalog No.:BCN3791
CAS No.:10323-20-3
- 1-Methyl-L-4,5-dihydroorotic acid
Catalog No.:BCC8472
CAS No.:103365-69-1
- GNE-493
Catalog No.:BCC8048
CAS No.:1033735-94-2
- Itol A
Catalog No.:BCN5847
CAS No.:1033747-78-2
- GNF-5837
Catalog No.:BCC3668
CAS No.:1033769-28-6
- Salidroside
Catalog No.:BCN5966
CAS No.:10338-51-9
- Telotristat
Catalog No.:BCC5128
CAS No.:1033805-28-5
- HPGDS inhibitor 1
Catalog No.:BCC4065
CAS No.:1033836-12-2
- 1-O-Methylnataloe-emodin
Catalog No.:BCN7036
CAS No.:103392-51-4
- Octyl gallate
Catalog No.:BCN8432
CAS No.:1034-01-1
- Disodium (R)-2-Hydroxyglutarate
Catalog No.:BCC6515
CAS No.:103404-90-6
- Devazepide
Catalog No.:BCC7319
CAS No.:103420-77-5
- CTOP
Catalog No.:BCC5780
CAS No.:103429-31-8
L-364,373 (R-L3) enantiomers have opposite modulating effects on IKs in mammalian ventricular myocytes.[Pubmed:23889560]
Can J Physiol Pharmacol. 2013 Aug;91(8):586-92.
Activators of the slow delayed rectifier K(+) current (IKs) have been suggested as promising tools for suppressing ventricular arrhythmias due to prolongation of repolarization. Recently, L-364,373 (R-L3) was nominated to activate IKs in myocytes from several species; however, in some studies, it failed to activate IKs. One later study suggested opposite modulating effects from the R-L3 enantiomers as a possible explanation for this discrepancy. Therefore, we analyzed the effect of the RL-3 enantiomers on IKs in ventricular mammalian myocytes, by applying standard microelectrode and whole-cell patch-clamp techniques at 37 degrees C. We synthesized 2 substances, ZS_1270B (right) and ZS_1271B (left), the 2 enantiomers of R-L3. In rabbit myocytes, ZS_1270B enhanced the IKs tail current by approximately 30%, whereas ZS_1271B reduced IKs tails by 45%. In guinea pig right ventricular preparations, ZS_1270B shortened APD90 (action potential duration measured at 90% repolarization) by 12%, whereas ZS_1271B lengthened it by approximately 15%. We concluded that R-L3 enantiomers in the same concentration range indeed have opposite modulating effects on IKs, which may explain why the racemic drug R-L3 previously failed to activate IKs. ZS_1270B is a potent IKs activator, therefore, this substance is appropriate to test whether IKs activators are ideal tools to suppress ventricular arrhythmias originating from prolongation of action potentials.
L-364,373 fails to activate the slow delayed rectifier K+ current in canine ventricular cardiomyocytes.[Pubmed:16544107]
Naunyn Schmiedebergs Arch Pharmacol. 2006 Apr;373(1):85-9.
Activators of the slow delayed rectifier K+ current (I(Ks)) are promising tools to suppress ventricular arrhythmias originating from prolongation of action potentials. A recently synthesized compound, L-364,373, was shown to activate I(Ks) in ventricular cells isolated from guinea pigs and rabbits. Due to the interspecies differences known to exist in the properties of the delayed rectifier K+ currents, the effect of L-364,373 on I(Ks) was studied and compared with that of another I(Ks) activator mefenamic acid in canine ventricular myocytes. Mefenamic acid (100 microM) significantly increased the amplitude of the fully activated I(Ks) current, as well as the I(Ks) current tails, by shifting the voltage dependence of its activation towards negative voltages and increased the time constant for deactivation. In contrast, L-364,373, up to concentrations of 3 microM, failed to augment I(Ks) at any membrane potential studied, but slightly increased the time constant of deactivation. It is concluded that human studies are required to evaluate the therapeutically beneficial effects of I(Ks) activators. Rodent cardiac tissues are not suitable for this purpose.
Rb+ efflux through functional activation of cardiac KCNQ1/minK channels by the benzodiazepine R-L3 (L-364,373).[Pubmed:16945016]
Assay Drug Dev Technol. 2006 Aug;4(4):443-50.
The slow delayed rectifier K+ current, Iks, encoded by KCNQ1 (KvLQT1)/KCNE1 (mink) genes, contributes to cardiac action potential repolarization and determines the heartbeat rate. Mutations in either KCNQ1 or KCNE1 that reduce Iks cause long-QT syndrome (LQTS), a disorder of ventricular repolarization that results in cardiac arrhythmia and sudden death. A well-recognized potential treatment for LQTS caused by reduction of Iks is to enhance functional activation of cardiac KCNQ1/KCNE1 channels. In the present study, we generated a stable Chinese hamster ovary cell line that expresses KCNQ1/KCNE1 channels confirmed by electrophysiology. Using a pharmacological tool compound R-L3 (L-364,373 [(3-R)-1,3-dihydro-5-(2-fluorophenyl)-3-(1H-indol- 3-ylmethyl)-1-methyl-2H-1,4-benzodiazepin-2-one]), which activates KCNQ1/mink channels, we then developed and validated a non-radioactive rubidium (Rb+) efflux assay that directly measures the functional activity of KCNQ1/KCNE1 channels by atomic absorption spectroscopy. Our results show that the validated Rb+ efflux assay can be used for screening of KCNQ1/KCNE1 openers that potentially treat LQTS in both inherited and acquired forms. In addition, the assay also can be used for evaluation of possible long-QT liability during cardiac selectivity of new chemical entities.
Pharmacological dissection of K(v)7.1 channels in systemic and pulmonary arteries.[Pubmed:22251082]
Br J Pharmacol. 2012 Jun;166(4):1377-87.
BACKGROUND AND PURPOSE: The aim of this study was to characterize the functional impact of KCNQ1-encoded voltage-dependent potassium channels (K(v)7.1) in the vasculature. EXPERIMENTAL APPROACH: Mesenteric arteries, intrapulmonary arteries and thoracic aortae were isolated from adult rats. K(v)7.1 channel expression was established by fluorescence immunocytochemistry. Wire myography determined functionality of these channels in response to selective blockers and activators. Xenopus oocytes expressing K(v)7.1 channels were used to assess the effectiveness of selective K(v)7.1 channel blockers. KEY RESULTS: K(v)7.1 channels were identified in arterial myocytes by immunocytochemistry. K(v)7.1 blockers HMR1556, L-768,673 (10 microM) and JNJ39490282 (JNJ282; 1 microM) had no contractile effects in arteries, whereas the pan-K(v)7 channel blocker linopirdine (10 microM) evoked robust contractions. Application of two compounds purported to activate K(v)7.1 channels, L-364 373 (R-L3) and mefenamic acid, relaxed mesenteric arteries preconstricted by methoxamine. These responses were reversed by HMR1556 or L-768,673 but not JNJ282. Similar effects were observed in the thoracic aorta and intrapulmonary arteries. CONCLUSIONS AND IMPLICATIONS: In contrast to previous assumptions, K(v)7.1 channels expressed in arterial myocytes are functional ion channels. Although these channels do not appear to contribute to resting vascular tone, K(v)7.1 activators were effective vasorelaxants.
Pharmacological activation of normal and arrhythmia-associated mutant KCNQ1 potassium channels.[Pubmed:14576198]
Circ Res. 2003 Nov 14;93(10):941-7.
KCNQ1 alpha-subunits coassemble with KCNE1 beta-subunits to form channels that conduct the slow delayed rectifier K+ current (IKs) important for repolarization of the cardiac action potential. Mutations in KCNQ1 reduce IKs and cause long-QT syndrome, a disorder of ventricular repolarization that predisposes affected individuals to arrhythmia and sudden death. Current therapy for long-QT syndrome is inadequate. R-L3 is a benzodiazepine that activates IKs and has the potential to provide gene-specific therapy. In the present study, we characterize the molecular determinants of R-L3 interaction with KCNQ1 channels, use computer modeling to propose a mechanism for drug-induced changes in channel gating, and determine its effect on several long-QT syndrome-associated mutant KCNQ1 channels heterologously expressed in Xenopus oocytes. Scanning mutagenesis combined with voltage-clamp analysis indicated that R-L3 interacts with specific residues located in the 5th and 6th transmembrane domains of KCNQ1 subunits. Most KCNQ1 mutant channels responded to R-L3 similarly to wild-type channels, but one mutant channel (G306R) was insensitive to R-L3 possibly because it disrupted a key component of the drug-binding site.


