HPGDS inhibitor 1HPGDS inhibitor CAS# 1033836-12-2 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1033836-12-2 | SDF | Download SDF |
| PubChem ID | 24991044 | Appearance | Powder |
| Formula | C19H19F4N3O | M.Wt | 381.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (131.11 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
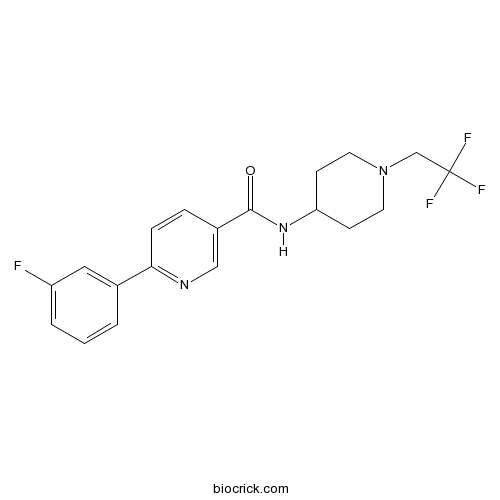
| Chemical Name | 6-(3-fluorophenyl)-N-[1-(2,2,2-trifluoroethyl)piperidin-4-yl]pyridine-3-carboxamide | ||
| SMILES | C1CN(CCC1NC(=O)C2=CN=C(C=C2)C3=CC(=CC=C3)F)CC(F)(F)F | ||
| Standard InChIKey | LPUCBGGXXIUBAZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H19F4N3O/c20-15-3-1-2-13(10-15)17-5-4-14(11-24-17)18(27)25-16-6-8-26(9-7-16)12-19(21,22)23/h1-5,10-11,16H,6-9,12H2,(H,25,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | HPGDS inhibitor 1 is a novel and selective Hematopoietic Prostaglandin D Synthase (HPGDS) inhibitor with an IC50 Value of 0.7 nM.
IC50 Value: 0.7 nM [1]
Target: HPGDS
HPGDS inhibitor 1 was elected for further profiling based on its enzyme and cell potency. The compound illustrated equal potency against purified HPGDS from human , rat, dog, and sheep (IC50, 0.5-2.3 nM). HPGDS inhibitor 1 was profiled in a panel of cellular assays to screen for activity against several relevant human enzyme targets. Those assay indicated that HPGDS inhibitor 1 does not inhibit human L- PGDS, m-PGDS, COX-1, COX-2 or 5 LOX (IC50 values > 10000 nM).
HPGDS inhibitor 1 had a solubility of 1.5 ug/ml (3.9 uM) at pH 6.5. The compound had excellent PK characteristics when dosed in rats at 1 mpk with 76% bioavailavility.
Rats dosed orally with 1 and 10 mpk HPGDS inhibitor 1 were sacrificed at various times, and plasma concentrations of HPGDS inhibitor 1 and spleen PGD2 concentrations were measured. Oral administration of HPGDS inhibitor 1 blocked PGD2 production in the rat spleen; inhibition of PGD2 was inversely correlated with the plasma concentration of HPGDS inhibitor 1 in a time and dose-dependent manner. Spleen PGD2 levels fall as HPGDS inhibitor 1 plasma levels increase over time; PGD2 levels return to baseline levels as HPGDS inhibitor 1 plasma levels decline. References: | |||||

HPGDS inhibitor 1 Dilution Calculator

HPGDS inhibitor 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6221 mL | 13.1106 mL | 26.2213 mL | 52.4425 mL | 65.5531 mL |
| 5 mM | 0.5244 mL | 2.6221 mL | 5.2443 mL | 10.4885 mL | 13.1106 mL |
| 10 mM | 0.2622 mL | 1.3111 mL | 2.6221 mL | 5.2443 mL | 6.5553 mL |
| 50 mM | 0.0524 mL | 0.2622 mL | 0.5244 mL | 1.0489 mL | 1.3111 mL |
| 100 mM | 0.0262 mL | 0.1311 mL | 0.2622 mL | 0.5244 mL | 0.6555 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
HPGDS inhibitor 1 is an oral potent and selective inhibitor of hematopoietic prostaglandin D synthase (HPGDS) with IC50 value of 0.7nM [1].
Prostaglandin D2 (PGD2) is a mediator of allergy and inflammation. It is isomerized from PGH2 by PGDS. This production is important in airway allergic and inflammatory processes. HPGDS inhibitor 1 has a 3-fluorine substituent, making it stable in the in vitro human microsome assay. It has potent effects in enzyme assay and cellular assay with IC50 values of 0.7nM and 32nM, respectively. In addition, the potency is equal against purified HPGDS from various species with IC50 values ranging from 0.5-2.3nM. Besides that, HPGDS inhibitor 1 is selective against HPGDS over other relevant human enzymes including L-PGDS, mPGES, COX-1, COX-2 and 5 LOX [1].
In a model of antigen-induced airway response in allergic sheep, treatment of HPGDS inhibitor 1 completely prevents the late asthma reaction and blocks the Airway hyper-responsiveness [1].
References:
[1] Carron C P, Trujillo J I, Olson K L, et al. Discovery of an oral potent selective inhibitor of hematopoietic prostaglandin D synthase (HPGDS). ACS Medicinal Chemistry Letters, 2010, 1(2): 59-63.
- Telotristat
Catalog No.:BCC5128
CAS No.:1033805-28-5
- Salidroside
Catalog No.:BCN5966
CAS No.:10338-51-9
- GNF-5837
Catalog No.:BCC3668
CAS No.:1033769-28-6
- Itol A
Catalog No.:BCN5847
CAS No.:1033747-78-2
- GNE-493
Catalog No.:BCC8048
CAS No.:1033735-94-2
- 1-Methyl-L-4,5-dihydroorotic acid
Catalog No.:BCC8472
CAS No.:103365-69-1
- L-364,373
Catalog No.:BCC7445
CAS No.:103342-82-1
- 3-Oxo-4-aza-5-alpha-androstane-17β-carboxylic acid
Catalog No.:BCC8641
CAS No.:103335-55-3
- Pre-schisanartanin B
Catalog No.:BCN5846
CAS No.:1033288-92-4
- Taltirelin
Catalog No.:BCC5271
CAS No.:103300-74-9
- LDK378
Catalog No.:BCC3691
CAS No.:1032900-25-6
- GSK1292263
Catalog No.:BCC3786
CAS No.:1032823-75-8
- 1-O-Methylnataloe-emodin
Catalog No.:BCN7036
CAS No.:103392-51-4
- Octyl gallate
Catalog No.:BCN8432
CAS No.:1034-01-1
- Disodium (R)-2-Hydroxyglutarate
Catalog No.:BCC6515
CAS No.:103404-90-6
- Devazepide
Catalog No.:BCC7319
CAS No.:103420-77-5
- CTOP
Catalog No.:BCC5780
CAS No.:103429-31-8
- CTAP
Catalog No.:BCC5776
CAS No.:103429-32-9
- Z-Cyclopentyl-AP4
Catalog No.:BCC7616
CAS No.:103439-17-4
- Xanthone V1a
Catalog No.:BCN7922
CAS No.:103450-96-0
- NMS-1286937
Catalog No.:BCC5358
CAS No.:1034616-18-6
- Maprotiline HCl
Catalog No.:BCC4329
CAS No.:10347-81-6
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- Diacetylpiptocarphol
Catalog No.:BCN4737
CAS No.:103476-99-9
Hematopoetic prostaglandin D synthase: an ESR1-dependent oviductal epithelial cell synthase.[Pubmed:22374975]
Endocrinology. 2012 Apr;153(4):1925-35.
Oviductal disease is a primary cause of infertility, a problem that largely stems from excessive inflammation of this key reproductive organ. Our poor understanding of the mechanisms regulating oviductal inflammation restricts our ability to diagnose, treat, and/or prevent oviductal disease. Using mice, our objective was to determine the spatial localization, regulatory mechanism, and functional attributes of a hypothesized regulator of oviductal inflammation, the hematopoietic form of prostaglandin D synthase (HPGDS). Immunohistochemistry revealed specific localization of HPGDS to the oviduct's epithelium. In the isthmus, expression of HPGDS was consistent. In the ampulla, expression of HPGDS appeared dependent upon stage of the estrous cycle. HPGDS was expressed in the epithelium of immature and cycling mice but not in the oviducts of estrogen receptor alpha knockouts. Two receptor subtypes bind PGD(2): PGD(2) receptor and G protein-coupled receptor 44. Expression of mRNA for Ptgdr was higher in the epithelial cells (EPI) than in the stroma (P < 0.05), whereas mRNA for Gpr44 was higher in the stroma than epithelium (P < 0.05). Treatment of human oviductal EPI with HQL-79, an inhibitor of HPGDS, decreased cell viability (P < 0.05). Treatment of mice with HQL-79 increased mRNA for chemokine (C-C motif) ligands 3, 4, and 19; chemokine (C-X-C motif) ligands 11 and 12; IL-13 and IL-17B; and TNF receptor superfamily, member 1b (P < 0.02 for each mRNA). Overall, these results suggest that HPGDS may play a role in the regulation of inflammation and EPI health within the oviduct.
Expression and detrimental role of hematopoietic prostaglandin D synthase in spinal cord contusion injury.[Pubmed:21294159]
Glia. 2011 Apr;59(4):603-14.
Prostaglandin D(2) (PGD(2) ) is a potent inflammatory mediator, which is implicated in both the initiation and resolution of inflammation in peripheral non-neural tissues. Its role in the central nervous system has not been fully elucidated. Spinal cord injury (SCI) is associated with an acute inflammatory response, which contributes to secondary tissue damage that worsens functional loss. We show here, with the use of hematopoietic prostaglandin D synthase (HPGDS) deficient mice and a HPGDS selective inhibitor (HQL-79), that PGD(2) plays a detrimental role after SCI. We also show that HPGDS is expressed in macrophages in the injured mouse spinal cord and contributes to the increase in PGD(2) in the contused spinal cord. HPGDS(-/-) mice also show reduced secondary tissue damage and reduced expression of the proinflammatory chemokine CXCL10 as well as an increase in IL-6 and TGFbeta-1 expression in the injured spinal cord. This was accompanied by a reduction in the expression of the microglia/macrophage activation marker Mac-2 and an increase in the antioxidant metallothionein III. Importantly, HPGDS deficient mice exhibit significantly better locomotor recovery after spinal cord contusion injury than wild-type (Wt) mice. In addition, systemically administered HPGDS inhibitor (HQL-79) also enhanced locomotor recovery after SCI in Wt mice. These data suggest that PGD(2) generated via HPGDS has detrimental effects after SCI and that blocking the activity of this enzyme can be beneficial.
Suppressive effects of antimycotics on thymic stromal lymphopoietin production in human keratinocytes.[Pubmed:23688403]
J Dermatol Sci. 2013 Sep;71(3):174-83.
BACKGROUND: Thymic stromal lymphopoietin (TSLP) is produced by epidermal keratinocytes, and it induces Th2-mediated inflammation. TSLP expression is enhanced in lesions with atopic dermatitis, and is a therapeutic target. Antimycotic agents improve the symptoms of atopic dermatitis. OBJECTIVE: The objective of this study was to examine whether antimycotics suppress TSLP expression in human keratinocytes. METHODS: Normal human keratinocytes were incubated with polyinosinic-polycytidylic acid (poly I:C) plus IL-4 in the presence of antimycotics. TSLP expression was analyzed by ELISA and real time PCR. Luciferase assays were performed to analyze NF-kappaB activity. IkappaBalpha degradation was analyzed by Western blot analysis. RESULTS: Poly I:C plus IL-4 increased the secretion and mRNA levels of TSLP, which was suppressed by an NF-kappaB inhibitor, and also enhanced NF-kappaB transcriptional activities and induced the degradation of IkappaBalpha in keratinocytes. The antimycotics itraconazole, ketoconazole, luliconazole, terbinafine, butenafine, and amorolfine suppressed the secretion and mRNA expression of TSLP, NF-kappaB activity, and IkappaBalpha degradation induced by poly I:C plus IL-4. These suppressive effects were similarly manifested by 15-deoxy-Delta-(12,14)-PGJ2 (15d-PGJ2), a prostaglandin D2 metabolite. Antimycotics increased the release of 15d-PGJ2 from keratinocytes and decreased the release of thromboxane B2, a thromboxane A2 metabolite. Antimycotic-induced suppression of TSLP production and NF-kappaB activity was counteracted by an inhibitor of lipocalin type-prostaglandin D synthase. CONCLUSIONS: Antimycotics itraconazole, ketoconazole, luliconazole, terbinafine, butenafine, and amorolfine may suppress poly I:C plus IL-4-induced production of TSLP by inhibiting NF-kappaB via increasing 15d-PGJ2 production in keratinocytes. These antimycotics may block the overexpression of TSLP in lesions with atopic dermatitis.
Up-regulation of the inflammatory response by ovariectomy in collagen-induced arthritis. effects of tin protoporphyrin IX.[Pubmed:21046213]
Inflammation. 2011 Dec;34(6):585-96.
We have studied the influence of ovariectomy on the inflammatory response and bone metabolism on CIA as a model of postmenopausal arthritis as well as the effects of tin protoporphyrin IX (SnPP), a heme oxygenase inhibitor. Ovariectomy in non-arthritic mice produced increased serum PGD2 levels and up-regulated the expression of COX-2, h-PGDS, l-PGDS, and HO-1 in the joints. In CIA, ovariectomy potentiated the inflammatory response with higher levels of serum IL-6 and MMP-3, local PGD2 and MMP-3 as well as trabecular bone erosion. In OVX-CIA, SnPP decreased the serum levels of IL-6, MMP-3, and PGD2; down-regulated TNFalpha, COX-2, hPGDS, PGD2, PGE2, and MMP-3 in joint tissues; and also decreased focal bone loss in the inflamed joint. Ovariectomy up-regulates inflammatory mediators in non-arthritic and in arthritic animals. In the OVX-CIA model, SnPP exerts anti-inflammatory effects which are not associated with the prevention of systemic bone loss.
Identification of new inhibitors for human hematopoietic prostaglandin D2 synthase among FDA-approved drugs and other compounds.[Pubmed:25603235]
Chem Biol Interact. 2015 Mar 5;229:91-9.
OBJECTIVE: Hematopoietic prostaglandin D2 synthase (HPGDS) is a member of the Sigma class glutathione transferases (GSTs) catalyzing the isomerization of prostaglandin H2 to prostaglandin D2, a mediator of allergy and inflammation responses. Selective inhibitors of human HPGDS are expected to be of therapeutic importance in relieving symptoms related to allergy and asthma. Hence, a collection of diverse FDA-approved compounds was screened for potential novel applications as inhibitors of HPGDS. METHODS: The catalytic activity of purified HPGDS was used for inhibition studies in vitro. RESULTS: Our inhibition studies revealed 23 compounds as effective inhibitors of HPGDS with IC50 values in the low micromolar range. Erythrosine sodium, suramin, tannic acid and sanguinarine sulfate were characterized with IC50 values of 0.2, 0.3, 0.4, and 0.6 muM, respectively. Kinetic inhibition analysis showed that erythrosine sodium is a nonlinear competitive inhibitor of HPGDS, while suramin, tannic acid and sanguinarine sulfate are linear competitive inhibitors. CONCLUSION: The results show that certain FDA-approved compounds may have pharmacological effects not previously realized that warrant further consideration in their clinical use.


