DevazepideCCK-A receptor antagonist, potent CAS# 103420-77-5 |
- A-71623
Catalog No.:BCC7354
CAS No.:130408-77-4
- SR 27897
Catalog No.:BCC7277
CAS No.:136381-85-6
- CCK Octapeptide, non-sulfated
Catalog No.:BCC5709
CAS No.:25679-24-7
- Proglumide sodium salt
Catalog No.:BCC5768
CAS No.:99247-33-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103420-77-5 | SDF | Download SDF |
| PubChem ID | 443375 | Appearance | Powder |
| Formula | C25H20N4O2 | M.Wt | 408.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | L-364,718, MK 329 | ||
| Solubility | Soluble to 100 mM in DMSO and to 50 mM in ethanol | ||
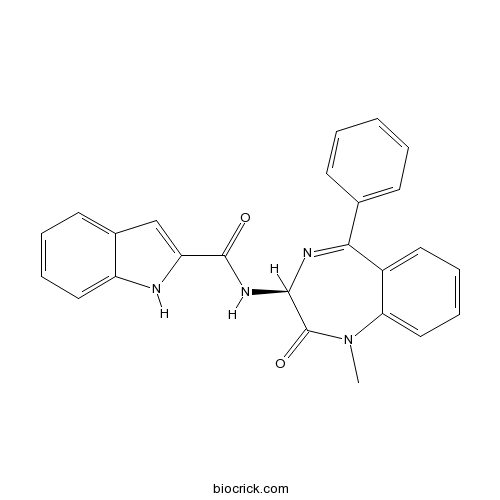
| Chemical Name | N-[(3S)-1-methyl-2-oxo-5-phenyl-3H-1,4-benzodiazepin-3-yl]-1H-indole-2-carboxamide | ||
| SMILES | CN1C2=CC=CC=C2C(=NC(C1=O)NC(=O)C3=CC4=CC=CC=C4N3)C5=CC=CC=C5 | ||
| Standard InChIKey | NFHRQQKPEBFUJK-HSZRJFAPSA-N | ||
| Standard InChI | InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, orally active CCK1 (CCK-A) receptor antagonist that displays appetite-stimulant effects. Blocks the anorectic response to CCK-8 and increases food intake in rats following systemic and i.c.v administration. |

Devazepide Dilution Calculator

Devazepide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4482 mL | 12.2411 mL | 24.4822 mL | 48.9644 mL | 61.2055 mL |
| 5 mM | 0.4896 mL | 2.4482 mL | 4.8964 mL | 9.7929 mL | 12.2411 mL |
| 10 mM | 0.2448 mL | 1.2241 mL | 2.4482 mL | 4.8964 mL | 6.1206 mL |
| 50 mM | 0.049 mL | 0.2448 mL | 0.4896 mL | 0.9793 mL | 1.2241 mL |
| 100 mM | 0.0245 mL | 0.1224 mL | 0.2448 mL | 0.4896 mL | 0.6121 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Devazepide is a potent CCK-A receptor antagonist with IC50 of 0.26 nM. [1]
Cholecystokinin (CCK) has long been recognized as the dominant regulator of gallbladder contraction. The cholecystokinetic activity of different fragments of CCK in human has been established in several studies both in vivo and in vitro. There are two subtypes of CCK receptor that were identified, CCK-A and CCK-B. CCK-A are mainly located in peripheral tissues but also found in brain, while CCK-B distribute throughout the rodent central nervous system.
Devazeptide inhibitory effect against sulphated C-terminal octapeptide of cholecystokinin (CCK-8) on total [3H] inositol phosphate accumulation in CHO-AI cells was investigated. The results revealed that devazepide possesses an inhibitory effect with IC50 of 0.26 ± 0.06 nM. CCK-8 is considered to be the most potent endogenous anti-opiate agent. Morphine analgesia could be antagonized by treatment of CCK-8. However, the effect of CCK-8 could be reversed by devazeptide which could also potentiate analgesia of morphine greatly at doses of 50ng and 200ng. [2, 3]
Devazepide is a benzodiazepine derivative that interacts competitively with rat pancreatic CCK receptors as determined by Scatchard analysis against the specific binding of 125I-labelled CCK. In guinea-pig isolated ileum and colon, devazepide competitively blocked CCK-induced contractions with a potency greatly exceeding that of other non-peptide CCK antagonists such as proglumide, dibutyryl cyclic GMP and benzotript. It is confirmed that devazepide is a highly potent CCK-OP antagonist in this tissue. [4]
References:
[1] Dickenson, John M. , and Stephen J. Hill. "Synergistic interactions between human transfected adenosine A 1 receptors and endogenous cholecystokinin receptors in CHO cells."?European journal of pharmacology?302.1 (1996): 141-151.
[2] Lavigne, G. J., W. R. Millington, and G. P. Mueller. "The CCK-A and CCK-B receptor antagonists, devazepide and L-365,260, enhance morphine antinociception only in non-acclimated rats exposed to a novel environment."Neuropeptides?21.2 (1992): 119-129.
[3] Pu, Su-Fen, Hui-Xin Zhuang, and Ji-Sheng Han. "Cholecystokinin octapeptide (CCK-8) antagonizes morphine analgesia in nucleus accumbens of the rat via the CCK-B receptor."?Brain research?657.1 (1994): 159-164.
[4] D'amato, M. , I. F. Stamford, and A. Bennett. "Studies of three non‐peptide cholecystokinin antagonists (devazepide, lorglumide and loxiglumide) in human isolated alimentary muscle and guinea‐pig ileum."?British journal of pharmacology?102.2 (1991): 391-395.
- Disodium (R)-2-Hydroxyglutarate
Catalog No.:BCC6515
CAS No.:103404-90-6
- Octyl gallate
Catalog No.:BCN8432
CAS No.:1034-01-1
- 1-O-Methylnataloe-emodin
Catalog No.:BCN7036
CAS No.:103392-51-4
- HPGDS inhibitor 1
Catalog No.:BCC4065
CAS No.:1033836-12-2
- Telotristat
Catalog No.:BCC5128
CAS No.:1033805-28-5
- Salidroside
Catalog No.:BCN5966
CAS No.:10338-51-9
- GNF-5837
Catalog No.:BCC3668
CAS No.:1033769-28-6
- Itol A
Catalog No.:BCN5847
CAS No.:1033747-78-2
- GNE-493
Catalog No.:BCC8048
CAS No.:1033735-94-2
- 1-Methyl-L-4,5-dihydroorotic acid
Catalog No.:BCC8472
CAS No.:103365-69-1
- L-364,373
Catalog No.:BCC7445
CAS No.:103342-82-1
- 3-Oxo-4-aza-5-alpha-androstane-17β-carboxylic acid
Catalog No.:BCC8641
CAS No.:103335-55-3
- CTOP
Catalog No.:BCC5780
CAS No.:103429-31-8
- CTAP
Catalog No.:BCC5776
CAS No.:103429-32-9
- Z-Cyclopentyl-AP4
Catalog No.:BCC7616
CAS No.:103439-17-4
- Xanthone V1a
Catalog No.:BCN7922
CAS No.:103450-96-0
- NMS-1286937
Catalog No.:BCC5358
CAS No.:1034616-18-6
- Maprotiline HCl
Catalog No.:BCC4329
CAS No.:10347-81-6
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- Diacetylpiptocarphol
Catalog No.:BCN4737
CAS No.:103476-99-9
- Fmoc-D-N- Me-Val-OH
Catalog No.:BCC3359
CAS No.:103478-58-6
- Fmoc-N-Me-Leu-OH
Catalog No.:BCC3345
CAS No.:103478-62-2
- Fmoc-D-N-Me-Leu-OH
Catalog No.:BCC3346
CAS No.:103478-63-3
- 9-Dehydroandrostenedione
Catalog No.:BCC8801
CAS No.:1035-69-4
Devazepide fails to reverse the inhibitory effect of interleukin-1beta on food intake in female rats.[Pubmed:15451641]
Physiol Behav. 2004 Oct 15;82(5):777-83.
Proinflammatory cytokines released during the course of infection elicit numerous behavioral and metabolic changes. The decrease in food intake that accompanies infection is mediated in part by interleukin-1 (IL-1). Cholecystokinin (CCK) is a neuropeptide released during a meal, decreases food intake, and previous research suggests that CCK mediates the anorectic action of IL-1. The effects of estrogen on food intake are also thought to involve CCK, as the satiety action of CCK is increased by estradiol in both intact and ovariectomized rats. Estradiol also modulates many of the behavioral and physiological effects of IL-1. The present experiment examined the ability of the CCK(A) receptor antagonist Devazepide to block the effects of IL-1 and estradiol on food intake in female rats. Adult animals were ovariectomized and given two daily subcutaneous injections of estradiol benzoate (EB; 5.0 microg) or the oil vehicle 3 weeks after surgery. Three days after treatment onset, animals were pretreated with Devazepide or its vehicle 30 min prior to intraperitoneal injections of IL-1beta (4.0 microg/kg) or saline given 1 h before light offset. Food and water intake was measured following 2 h of spontaneous feeding. The results indicate that Devazepide failed to reverse the anorectic action of IL-1beta, although the effects of estradiol on food intake were blocked by Devazepide. These data do not support a role for CCK in IL-1-induced anorexia, and suggest that cytokines may act directly on neural systems involved in the control of food intake.
Devazepide, a nonpeptide antagonist of CCK receptors, induces apoptosis and inhibits Ewing tumor growth.[Pubmed:19407653]
Anticancer Drugs. 2009 Aug;20(7):527-33.
The Ewing family of tumors is a group of highly malignant tumors that mainly arise in bone and most often affect children and young adults in the first two decades of life. Despite the use of multimodal therapy, the long-term disease-free survival rate of patients with Ewing tumors is still disappointingly low, making the discovery of innovative therapeutic strategies all the more necessary. We have recently shown that cholecystokinin (CCK), a neuroendocrine peptide, involved in many biological functions, including cell growth and proliferation, is a relevant target of the EWS/FLI1 oncoprotein characteristic of Ewing tumors. CCK silencing inhibits cell proliferation and tumor growth in vivo, suggesting that CCK acts as an autocrine growth factor for Ewing cells. Here, we analyzed the impact of two CCK receptor antagonists, Devazepide (a CCK1-R antagonist) and L365 260 (a CCK2-R antagonist), on the growth of Ewing tumor cells. Devazepide (10 micromol/l) inhibited cell growth of four different Ewing tumor cells in vitro (range 85-88%), whereas the effect of the CCK2-R antagonist on cell growth was negligible. In a mouse tumor xenograft model, Devazepide reduced tumor growth by 40%. Flow cytometry experiments showed that Devazepide, but not L365 260, induced apoptosis of Ewing tumor cells. In summary, Devazepide induces cell death of Ewing tumor cells, suggesting that it could represent a new therapeutic approach in the management of Ewing's tumor patients.
Effects of intracerebroventricular administration of the CCK(1) receptor antagonist devazepide on food intake in rats.[Pubmed:12007923]
Eur J Pharmacol. 2002 Apr 19;441(1-2):79-82.
The effects of intracerebroventricular administration of Devazepide, a CCK(1) receptor antagonist, was investigated on food intake in rats. In the first experiment, rats (n=5) were deprived of food for 17 h and injected intracerebroventricularly with either vehicle or Devazepide (1, 10, 25 or 100 ng). Five minutes after vehicle or drug administration, the animals were presented with food and intake measured for 60 min. Devazepide produced a dose-related increase in food intake. Doses of 1, 10 and 25 ng significantly increased consumption (at least P<0.01 in each case). A second experiment was subsequently undertaken to investigate whether systemic administration of the intracerebroventricular doses used in the first experiment would affect food intake. Rats (n=8) that have been deprived of food for 17 h were injected intraperitoneally with either vehicle or Devazepide (3, 30, 75 or 300 ng/kg). Five minutes after vehicle or drug administration, the animals were presented with food and intake was measured for 60 min. Devazepide (3-300 ng/kg, i.p.) had no significant effects on food consumption. The results show that central administration of low doses of Devazepide increase food intake in rats, while similar doses, given systemically, do not affect consumption. These findings suggest the possibility that endogenous cholecystokinin (CCK), acting at central CCK(1) receptors, may play a physiological role in the control of feeding behaviour in the rat.
The cholecystokinin-1 receptor antagonist devazepide increases cholesterol cholelithogenesis in mice.[Pubmed:26683129]
Eur J Clin Invest. 2016 Feb;46(2):158-69.
BACKGROUND: A defect in gallbladder contraction function plays a key role in the pathogenesis of gallstones. The cholecystokinin-1 receptor (CCK-1R) antagonists have been extensively investigated for their therapeutic effects on gastrointestinal and metabolic diseases in animal studies and clinical trials. However, it is still unknown whether they have a potential effect on gallstone formation. DESIGN: To study whether the CCK-1R antagonists enhance cholelithogenesis, we investigated cholesterol crystallization, gallstone formation, hepatic lipid secretion, gallbladder emptying function and intestinal cholesterol absorption in male C57BL/6J mice treated by gavage with Devazepide (4 mg/day/kg) or vehicle (as controls) twice per day and fed the lithogenic diet for 21 days. RESULTS: During 21 days of feeding, oral administration of Devazepide significantly accelerated cholesterol crystallization and crystal growth to microlithiasis, with 40% of mice forming gallstones, whereas only agglomerated cholesterol monohydrate crystals were found in mice receiving vehicle. Compared to the vehicle group, fasting and postprandial residual gallbladder volumes in response to the high-fat meal were significantly larger in the Devazepide group during cholelithogenesis, showing reduced gallbladder emptying and bile stasis. Moreover, Devazepide significantly increased hepatic secretion of biliary cholesterol, but not phospholipids or bile salts. The percentage of intestinal cholesterol absorption was higher in Devazepide-treated mice, increasing the bioavailability of chylomicron-derived cholesterol in the liver for biliary hypersecretion into bile. These abnormalities induced supersaturated bile and rapid cholesterol crystallization. CONCLUSIONS: The potent CCK-1R antagonist Devazepide increases susceptibility to gallstone formation by impairing gallbladder emptying function, disrupting biliary cholesterol metabolism and enhancing intestinal cholesterol absorption in mice.
Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats.[Pubmed:25849133]
Nat Med. 2015 May;21(5):506-11.
Metformin is a first-line therapeutic option for the treatment of type 2 diabetes, even though its underlying mechanisms of action are relatively unclear. Metformin lowers blood glucose levels by inhibiting hepatic glucose production (HGP), an effect originally postulated to be due to a hepatic AMP-activated protein kinase (AMPK)-dependent mechanism. However, studies have questioned the contribution of hepatic AMPK to the effects of metformin on lowering hyperglycemia, and a gut-brain-liver axis that mediates intestinal nutrient- and hormone-induced lowering of HGP has been identified. Thus, it is possible that metformin affects HGP through this inter-organ crosstalk. Here we show that intraduodenal infusion of metformin for 50 min activated duodenal mucosal Ampk and lowered HGP in a rat 3 d high fat diet (HFD)-induced model of insulin resistance. Inhibition of duodenal Ampk negated the HGP-lowering effect of intraduodenal metformin, and both duodenal glucagon-like peptide-1 receptor (Glp-1r)-protein kinase A (Pka) signaling and a neuronal-mediated gut-brain-liver pathway were required for metformin to lower HGP. Preabsorptive metformin also lowered HGP in rat models of 28 d HFD-induced obesity and insulin resistance and nicotinamide (NA)-streptozotocin (STZ)-HFD-induced type 2 diabetes. In an unclamped setting, inhibition of duodenal Ampk reduced the glucose-lowering effects of a bolus metformin treatment in rat models of diabetes. These findings show that, in rat models of both obesity and diabetes, metformin activates a previously unappreciated duodenal Ampk-dependent pathway to lower HGP and plasma glucose levels.
Effects of peripheral CCK receptor blockade on food intake in rats.[Pubmed:12738611]
Am J Physiol Regul Integr Comp Physiol. 2003 Aug;285(2):R429-37.
Type A cholecystokinin receptor (CCKAR) antagonists differing in blood-brain barrier permeability were used to test the hypothesis that satiety is mediated, in part, by CCK action at CCKARs located peripheral to the blood-brain barrier. At dark onset, non-food-deprived rats received a bolus injection of Devazepide (2.5 micromol/kg iv), a 3-h infusion of A-70104 (1 or 3 micromol x kg-1 x h-1 iv), or vehicle either alone or coadministered with a 3-h infusion of CCK-8 (10 nmol x kg-1 x h-1 iv) or a 2-h intragastric infusion of peptone (1 g/h). Food intake was determined from continuous computer recordings of changes in food bowl weight. Devazepide penetrates the blood-brain barrier; A-70104, the dicyclohexylammonium salt of Nalpha-3-quinolinoyl-d-Glu-N,N-dipentylamide (A-65186), does not. CCK-8 inhibited 3-h food intake by more than 50% and both A-70104 and Devazepide abolished this response. A-70104 and Devazepide stimulated food intake and similarly attenuated the anorexic response to intragastric infusion of peptone. Thus endogenous CCK appears to act, in part, at CCKARs peripheral to the blood-brain barrier to inhibit food intake.


