SR 27897CAS# 136381-85-6 |
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- Clofazimine
Catalog No.:BCC4651
CAS No.:2030-63-9
- 5-hydroxypyrazine-2-carboxylic acid
Catalog No.:BCC1311
CAS No.:34604-60-9
- Nitazoxanide
Catalog No.:BCC3824
CAS No.:55981-09-4
- Sodium 4-Aminosalicylate
Catalog No.:BCC4609
CAS No.:6018-19-5
- Rifapentine
Catalog No.:BCC4937
CAS No.:61379-65-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 136381-85-6 | SDF | Download SDF |
| PubChem ID | 122077 | Appearance | Powder |
| Formula | C20H14ClN3O3S | M.Wt | 411.86 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Lintitript | ||
| Solubility | Soluble to 50 mM in DMSO | ||
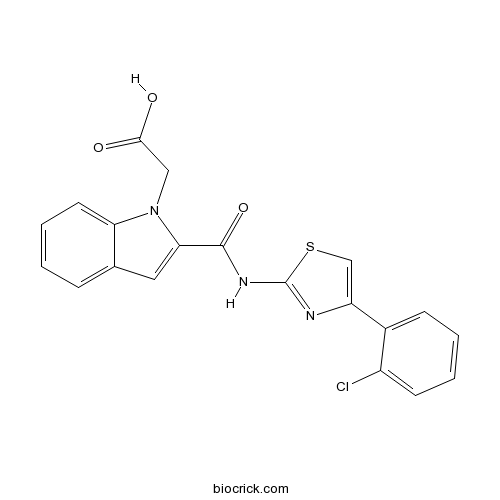
| Chemical Name | 2-[2-[[4-(2-chlorophenyl)-1,3-thiazol-2-yl]carbamoyl]indol-1-yl]acetic acid | ||
| SMILES | C1=CC=C2C(=C1)C=C(N2CC(=O)O)C(=O)NC3=NC(=CS3)C4=CC=CC=C4Cl | ||
| Standard InChIKey | ILNRQFBVVQUOLP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H14ClN3O3S/c21-14-7-3-2-6-13(14)15-11-28-20(22-15)23-19(27)17-9-12-5-1-4-8-16(12)24(17)10-18(25)26/h1-9,11H,10H2,(H,25,26)(H,22,23,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, non-peptide CCK1 receptor antagonist that displays > 33-fold selectivity over CCK2 receptors (EC50 values are 6 and 200 nM respectively). Causes an increase in plasma leptin levels and increases food intake in rats in vivo. |

SR 27897 Dilution Calculator

SR 27897 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.428 mL | 12.14 mL | 24.2801 mL | 48.5602 mL | 60.7002 mL |
| 5 mM | 0.4856 mL | 2.428 mL | 4.856 mL | 9.712 mL | 12.14 mL |
| 10 mM | 0.2428 mL | 1.214 mL | 2.428 mL | 4.856 mL | 6.07 mL |
| 50 mM | 0.0486 mL | 0.2428 mL | 0.4856 mL | 0.9712 mL | 1.214 mL |
| 100 mM | 0.0243 mL | 0.1214 mL | 0.2428 mL | 0.4856 mL | 0.607 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isophysalin A
Catalog No.:BCN7916
CAS No.:1363398-67-7
- Tiotropium Bromide
Catalog No.:BCC2000
CAS No.:136310-93-5
- Methyl 3-amino-2-[[(2'-cyanobiphenyl-4-yl)methyl]amino]benzoate
Catalog No.:BCC9037
CAS No.:136304-78-4
- Ethyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC8965
CAS No.:136285-67-1
- Ethyl2-((tert-butoxycarbonyl)amino)-3-nitrobenzoate
Catalog No.:BCC8978
CAS No.:136285-65-9
- Boc-ß-HoArg(Tos)-OH
Catalog No.:BCC3227
CAS No.:136271-81-3
- TAT 14
Catalog No.:BCC6295
CAS No.:1362661-34-4
- GNE-617
Catalog No.:BCC4280
CAS No.:1362154-70-8
- Absinthiin
Catalog No.:BCN2314
CAS No.:1362-42-1
- KW 3902
Catalog No.:BCC6124
CAS No.:136199-02-5
- 3,4'-Dihydroxy-3',5'-dimethoxypropiophenone
Catalog No.:BCN1579
CAS No.:136196-47-9
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- Duloxetine HCl
Catalog No.:BCC3773
CAS No.:136434-34-9
- YH239-EE
Catalog No.:BCC5454
CAS No.:1364488-67-4
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- Abacavir
Catalog No.:BCC1325
CAS No.:136470-78-5
- 11β-Hydroxy-2'-methyl-5'βH-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione
Catalog No.:BCC8435
CAS No.:13649-88-2
- Rink Amide Resin
Catalog No.:BCC2570
CAS No.:13653-84-4
- BQ-123
Catalog No.:BCC6963
CAS No.:136553-81-6
- Fmoc-D-Trp-OPfp
Catalog No.:BCC3560
CAS No.:136554-94-4
- Anemarsaponin E
Catalog No.:BCN6290
CAS No.:136565-73-6
- Curdione
Catalog No.:BCN5936
CAS No.:13657-68-6
- Irinotecan HCl Trihydrate
Catalog No.:BCC5091
CAS No.:136572-09-3
- Spermine NONOate
Catalog No.:BCC6950
CAS No.:136587-13-8
The agonist SR 146131 and the antagonist SR 27897 occupy different sites on the human CCK(1) receptor.[Pubmed:10988332]
Eur J Pharmacol. 2000 Jul 21;400(2-3):185-94.
1-[2-(4-(2-Chlorophenyl)thiazol-2-yl) aminocarbonyl indoyl] acetic acid (SR 27897) is an effective CCK(1) receptor antagonist, while the structurally related molecule 2-[4-(4-chloro-2, 5-dimethoxyphenyl)-5-(2-cyclohexyl-ethyl)-thiazol-2-ylcarbamoyl ]-5, 7-dimethyl-indol-1-yl-1-acetic acid (SR 146131) is a highly potent and specific agonist for the same receptor. To discover how the two molecules interact with the human cholecystokinin (CCK) CCK(1) receptor, we have carried out binding and activity studies with 33-point mutated receptors. Only six mutants showed altered [3H]SR 27897 binding properties, Lys(115), Lys(187), Phe(198), Trp(209), Leu(214) and Asn(333). In contrast, numerous mutations throughout the receptor either reduced SR 146131 agonist potency, Phe(97), Gly(122), Phe(198), Trp(209), Ile(229), Asn(333), Arg(336) and Leu(356) or increased it, Tyr(48), Cys(94), Asn(98), Leu(217) and Ser(359). Only mutations of Phe(198), Trp(209) and Asn(333) affected both SR 27897 and SR 146131 binding or activity. The collated information was used to construct molecular models of SR 27897 and SR 146131 bound to the human CCK(1) receptor. The clear difference in the binding sites of SR 27897 and SR 146131 offers a molecular explanation for their contrasting pharmacological characteristics.
Contrasting roles of leu(356) in the human CCK(1) receptor for antagonist SR 27897 and agonist SR 146131 binding.[Pubmed:10594328]
Eur J Pharmacol. 1999 Nov 3;383(3):339-46.
A new highly specific, potent non-peptide agonist for the cholecystokinin subtype 1 receptor (CCK(1)), SR 146131 (2-[4-(4-chloro-2, 5-dimethoxyphenyl)-5-(2-cyclohexyl-ethyl)-thiazol-2-ylcarbamoyl ]-5, 7-dimethyl-indol-1-yl-1-acetic acid) was recently described [Bignon, E., Bachy, A., Boigegrain, R., Brodin, R., Cottineau, M., Gully, D., Herbert, J.-M., Keane, P., Labie, C., Molimard, J.-C., Olliero, D., Oury-Donat, F., Petereau, C., Prabonneaud, V., Rockstroh, M.-P., Schaeffer, P., Servant, O.Thurneyssen, O., Soubrie, P., Pascal, M., Maffrand, J.-P., Le Fur, G., 1999. SR 146131: a new, potent, orally active and selective non-peptide cholecystokinin subtype I receptor agonist: I. In vitro studies. J. Pharmacol. Exp. Ther. 289, 742-751]. From binding and activity assays with chimeric constructs of human CCK(1) and the cholecystokinin subtype 2 receptor (CCK(2)) and receptors carrying point mutations, we show that Leu(356), situated in transmembrane domain seven in the CCK(1) receptor, is a putative contact point for SR 146131. In contrast, Leu(356) is probably not in contact with the CCK(1) receptor specific antagonist SR 27897 (1-[2-(4-(2-chlorophenyl)thiazol-2-yl)aminocarbonyl indoyl]acetic acid), a compound structurally related to SR 146131, since its replacement by alanine, histidine or asparagine gave receptors having wild-type CCK(1) receptor SR 27897 binding affinity. Previous mutational analysis of His(381), the cognate position in the rat CCK(2) receptor, had implicated it as being involved in subtype specificity for SR 27897, results which we confirm with corresponding mutations in the human CCK(2) receptor. Moreover, binding and activity assays with the natural CCK receptor agonist, CCK-8S, show that CCK-8S is more susceptible to the mutations in that position in the CCK(1) receptor than in the CCK(2) receptor. The results suggest different binding modes for SR 27897, SR 146131 and CCK-8S in each CCK receptor subtype.
Essential role of extracellular charged residues of the human CCK(1) receptor for interactions with SR 146131, SR 27897 and CCK-8S.[Pubmed:10688974]
Eur J Pharmacol. 2000 Feb 18;389(2-3):115-24.
We hypothesized that charge-charge interactions may be important for the binding of the human cholecystokinin type 1 (CCK(1)) receptor-specific non-peptide full agonist SR 146131, (2-[4-(4-chloro-2, 5-dimethoxyphenyl)-5-(2-cyclohexyl-ethyl)-thiazol-2-ylcarbamoyl ]-5, 7-dimethyl-indol-1-yl-1-acetic acid), the competitive antagonist SR 27897, (1-[2-(4-(2-chlorophenyl)thiazol-2-yl) aminocarbonyl indoyl] acetic acid) and the natural octapeptide CCK-8S to the CCK(1) receptor. Alanine replacement studies of positively charged residues in the extracellular domains of the receptor showed that only the R336A mutation affected SR 146131 potency of mutated receptors transiently expressed in monkey kidney epithelial COS-7 cells. Two residues, Lys(115) and Lys(187), were implicated in SR 27897 binding. Only the replacement of Lys(115), Arg(197) and Arg(336) significantly affected CCK-8S binding or activity. These results clearly indicated the importance of certain charged residues, but not others, in SR 146131, SR 27897 and CCK-8S binding. Furthermore, although these molecules probably occupy different binding sites on the CCK(1) receptor, we show that a small non-peptide agonist, SR 146131, can stimulate the dual signaling pathways mediated by the CCK(1) receptor.
Regulation of leptin distribution between plasma and cerebrospinal fluid by cholecystokinin receptors.[Pubmed:14534148]
Br J Pharmacol. 2003 Oct;140(4):647-52.
Cholecystokinin (CCK) is a postprandial hormone that elicits a satiating effect and regulates feeding behaviour. CCK has been shown to enhance the effect of leptin in several experimental paradigms. The goal of this work was to characterize the effect of endogenous CCK on plasma leptin content by using CCK receptor antagonists. Therefore, we administered SR-27897, a selective CCK1 receptor antagonist, and L-365260, a selective CCK2 receptor antagonist, to fed and food-deprived rats and determined plasma leptin concentration by enzyme immunoassay. Plasma insulin and glucose concentration as well as food intake were also determined. Under our conditions, SR-27897 increased plasma concentration of leptin both in fed and food-deprived rats. It also increased food intake as well as plasma concentration of insulin in fed animals. L-365260 increased plasma leptin concentration only in fed rats. In animals receiving exogenous leptin, CCK-8 increased the ratio between the concentration of leptin in cerebrospinal fluid and plasma. These results show that CCK receptor antagonists increases plasma concentration of leptin and suggest that endogenous CCK may facilitate the uptake of plasma leptin to the cerebrospinal fluid.


