TAT 14Nrf2 pathway activator; blocks Nrf2/Keap1 interaction CAS# 1362661-34-4 |
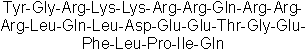
- Quercetin
Catalog No.:BCN6049
CAS No.:117-39-5
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- MEK inhibitor
Catalog No.:BCC1738
CAS No.:334951-92-7
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1362661-34-4 | SDF | Download SDF |
| PubChem ID | 60150581 | Appearance | Powder |
| Formula | C137H230N48O39 | M.Wt | 3173.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
| Sequence | YGRKKRRQRRRLQLDEETGEFLPIQ | ||
| SMILES | CCC(C)C(C(=O)NC(CCC(=O)N)C(=O)O)NC(=O)C1CCCN1C(=O)C(CC(C)C)NC(=O)C(CC2=CC=CC=C2)NC(=O)C(CCC(=O)O)NC(=O)CNC(=O)C(C(C)O)NC(=O)C(CCC(=O)O)NC(=O)C(CCC(=O)O)NC(=O)C(CC(=O)O)NC(=O)C(CC(C)C)NC(=O)C(CCC(=O)N)NC(=O)C(CC(C)C)NC(=O)C(CCCNC(=N)N)NC(=O)C(CCCNC(=N)N)NC(=O)C(CCCNC(=N)N)NC(=O)C(CCC(=O)N)NC(=O)C(CCCNC(=N)N)NC(=O)C(CCCNC(=N)N)NC(=O)C(CCCCN)NC(=O)C(CCCCN)NC(=O)C(CCCNC(=N)N)NC(=O)CNC(=O)C(CC3=CC=C(C=C3)O)N | ||
| Standard InChIKey | NBFQPSCRGQGZEP-YBKRDZSWSA-N | ||
| Standard InChI | InChI=1S/C137H230N48O39/c1-10-72(8)107(129(221)177-91(131(223)224)42-48-100(143)190)183-127(219)97-35-24-60-185(97)130(222)96(63-71(6)7)182-125(217)94(65-74-25-12-11-13-26-74)180-118(210)86(43-49-103(193)194)165-102(192)68-163-128(220)108(73(9)186)184-122(214)90(45-51-105(197)198)174-120(212)89(44-50-104(195)196)176-126(218)95(66-106(199)200)181-124(216)93(62-70(4)5)179-121(213)88(41-47-99(142)189)175-123(215)92(61-69(2)3)178-117(209)85(34-23-59-161-137(154)155)171-114(206)82(31-20-56-158-134(148)149)169-115(207)83(32-21-57-159-135(150)151)172-119(211)87(40-46-98(141)188)173-116(208)84(33-22-58-160-136(152)153)170-113(205)81(30-19-55-157-133(146)147)168-112(204)80(28-15-17-53-139)167-111(203)79(27-14-16-52-138)166-110(202)78(29-18-54-156-132(144)145)164-101(191)67-162-109(201)77(140)64-75-36-38-76(187)39-37-75/h11-13,25-26,36-39,69-73,77-97,107-108,186-187H,10,14-24,27-35,40-68,138-140H2,1-9H3,(H2,141,188)(H2,142,189)(H2,143,190)(H,162,201)(H,163,220)(H,164,191)(H,165,192)(H,166,202)(H,167,203)(H,168,204)(H,169,207)(H,170,205)(H,171,206)(H,172,211)(H,173,208)(H,174,212)(H,175,215)(H,176,218)(H,177,221)(H,178,209)(H,179,213)(H,180,210)(H,181,216)(H,182,217)(H,183,219)(H,184,214)(H,193,194)(H,195,196)(H,197,198)(H,199,200)(H,223,224)(H4,144,145,156)(H4,146,147,157)(H4,148,149,158)(H4,150,151,159)(H4,152,153,160)(H4,154,155,161)/t72-,73+,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,107-,108-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nrf2 activator; inhibits Nrf2/Keap1 interaction. Induces upregulation of Nrf2 pathway downstream gene expression including heme-oxygenase 1. Suppresses LPS-induced TNF-α expression in THP-1 cells. |

TAT 14 Dilution Calculator

TAT 14 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GNE-617
Catalog No.:BCC4280
CAS No.:1362154-70-8
- Absinthiin
Catalog No.:BCN2314
CAS No.:1362-42-1
- KW 3902
Catalog No.:BCC6124
CAS No.:136199-02-5
- 3,4'-Dihydroxy-3',5'-dimethoxypropiophenone
Catalog No.:BCN1579
CAS No.:136196-47-9
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- PALDA
Catalog No.:BCC7287
CAS No.:136181-87-8
- Neosarranicine
Catalog No.:BCN2024
CAS No.:136173-27-8
- Neosarracine
Catalog No.:BCN2026
CAS No.:136173-26-7
- Sarranicine
Catalog No.:BCN2025
CAS No.:136173-25-6
- 6-O-Caffeoylarbutin
Catalog No.:BCN6192
CAS No.:136172-60-6
- Lobetyol
Catalog No.:BCN3321
CAS No.:136171-87-4
- INNO-206
Catalog No.:BCC1651
CAS No.:1361644-26-9
- Boc-ß-HoArg(Tos)-OH
Catalog No.:BCC3227
CAS No.:136271-81-3
- Ethyl2-((tert-butoxycarbonyl)amino)-3-nitrobenzoate
Catalog No.:BCC8978
CAS No.:136285-65-9
- Ethyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC8965
CAS No.:136285-67-1
- Methyl 3-amino-2-[[(2'-cyanobiphenyl-4-yl)methyl]amino]benzoate
Catalog No.:BCC9037
CAS No.:136304-78-4
- Tiotropium Bromide
Catalog No.:BCC2000
CAS No.:136310-93-5
- Isophysalin A
Catalog No.:BCN7916
CAS No.:1363398-67-7
- SR 27897
Catalog No.:BCC7277
CAS No.:136381-85-6
- Duloxetine HCl
Catalog No.:BCC3773
CAS No.:136434-34-9
- YH239-EE
Catalog No.:BCC5454
CAS No.:1364488-67-4
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- Abacavir
Catalog No.:BCC1325
CAS No.:136470-78-5
- 11β-Hydroxy-2'-methyl-5'βH-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione
Catalog No.:BCC8435
CAS No.:13649-88-2
Induction of androgen formation in the male by a TAT-VDAC1 fusion peptide blocking 14-3-3varepsilon protein adaptor and mitochondrial VDAC1 interactions.[Pubmed:24947306]
Mol Ther. 2014 Oct;22(10):1779-91.
Low testosterone (T), a major cause of male hypogonadism and infertility, is linked to mood changes, fatigue, osteoporosis, reduced bone-mass index, and aging. The treatment of choice, T replacement therapy, has been linked with increased risk for prostate cancer and luteinizing hormone (LH) suppression, and shown to lead to infertility, cardiovascular diseases, and obesity. Alternate methods to induce T with lower side effects are desirable. In search of the mechanisms regulating T synthesis in the testes, we identified the 14-3-3varepsilon protein adaptor as a negative regulator of steroidogenesis. Steroidogenesis begins in mitochondria. 14-3-3varepsilon interacts with the outer mitochondrial membrane voltage-dependent anion channel (VDAC1) protein, forming a scaffold that limits the availability of cholesterol for steroidogenesis. We report the development of a tool able to induce endogenous T formation. Peptides able to penetrate testes conjugated to 14-3-3varepsilon site of interaction with VDAC1 blocked 14-3-3varepsilon-VDAC1 interactions while at the same time increased VDAC1-translocator protein (18 kDa) interactions that induced steroid formation in rat testes, leading to increased serum T levels. These peptides rescued intratesticular and serum T formation in adult male rats treated with gonadotropin-releasing hormone antagonist, which dampened LH and T production.
15-Deoxy-Delta12,14-prostaglandin J2 inhibits HIV-1 transactivating protein, Tat, through covalent modification.[Pubmed:19299483]
FASEB J. 2009 Aug;23(8):2366-73.
Controlling the HIV/AIDS epidemic remains a major challenge, with approximately 5 million new HIV infections annually. Cyclopentenone prostaglandins (CyPG), such as 15-deoxy-Delta(12,14)-PGJ(2) (15d-PGJ(2)), are arachidonic acid-derived endogenous electrophiles that possess anti-HIV activity by an unknown mechanism. Given that the reactive alpha,beta-unsaturated ketone in the cyclopentenone ring of 15d-PGJ(2) covalently modifies key Cys thiols in select proteins, we hypothesized that 15d-PGJ(2) inhibits HIV transcription and replication by targeting Cys thiols in HIV-1 Tat. Tat is a potent transactivator of viral gene expression required for HIV transcriptional elongation and replication. Our studies indicate that 15d-PGJ(2) treatment of cells inhibits Tat-dependent transcription and replication of HIV-1, while 9,10-dihydro-15d-PGJ(2), PGE(2), PGF(2alpha), or PGD(2) that lack the reactive alpha,beta-unsaturated ketone were ineffective. The inhibition of Tat activity by 15d-PGJ(2) was dose-dependent, with an IC(50) of 1.2 microM and independent of NF-kappaB pathway. Furthermore, using a biotinylated derivative of 15d-PGJ(2), we demonstrate that 15d-PGJ(2) modifies free Cys-thiols in Tat to form covalent Michael adducts and that the interaction was further increased on reduction of Tat. 15d-PGJ(2)-modified Tat was unable to transactivate the HIV long terminal repeat in U937 human macrophages. These data demonstrate that Tat acts as a molecular target of CyPG leading to the inhibition of transcription and also suggest a novel therapeutic approach to complement current antiretroviral strategies for HIV/AIDS.
Neuroprotective effect of TAT-14-3-3epsilon fusion protein against cerebral ischemia/reperfusion injury in rats.[Pubmed:24671253]
PLoS One. 2014 Mar 26;9(3):e93334.
Stroke is the major cause of death and disability worldwide, and the thrombolytic therapy currently available was unsatisfactory. 14-3-3epsilon is a well characterized member of 14-3-3 family, and has been reported to protect neurons against apoptosis in cerebral ischemia. However, it cannot transverse blood brain barrier (BBB) due to its large size. A protein transduction domain (PTD) of HIV TAT protein, is capable of delivering a large variety of proteins into the brain. In this study, we generated a fusion protein TAT-14-3-3epsilon, and evaluated its potential neuroprotective effect in rat focal ischemia/reperfusion (I/R) model. Western blot analysis validated the efficient transduction of TAT-14-3-3epsilon fusion protein into brain via a route of intravenous injection. TAT-14-3-3epsilon pre-treatment 2 h before ischemia significantly reduced cerebral infarction volume and improved neurologic score, while post-treatment 2 h after ischemia was less effective. Importantly, pre- or post-ischemic treatment with TAT-14-3-3epsilon significantly increased the number of surviving neurons as determined by Nissl staining, and attenuated I/R-induced neuronal apoptosis as showed by the decrease in apoptotic cell numbers and the inhibition of caspase-3 activity. Moreover, the introduction of 14-3-3epsilon into brain by TAT-mediated delivering reduced the formation of autophagosome, attenuated LC3B-II upregulation and reversed p62 downregulation induced by ischemic injury. Such inhibition of autophagy was reversed by treatment with an autophagy inducer rapamycin (RAP), which also attenuated the neuroprotective effect of TAT-14-3-3epsilon. Conversely, autophagy inhibitor 3-methyladenine (3-MA) inhibited I/R-induced the increase in autophagic activity, and attenuated I/R-induced brain infarct. These results suggest that TAT-14-3-3epsilon can be efficiently transduced into brain and exert significantly protective effect against brain ischemic injury through inhibiting neuronal apoptosis and autophagic activation.
Anti-inflammatory Effect of a Cell-Penetrating Peptide Targeting the Nrf2/Keap1 Interaction.[Pubmed:22582137]
ACS Med Chem Lett. 2012 May 10;3(5):407-410.
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is increasingly recognized as a central regulator of multiple signaling pathways in inflammation and cancer, and the ability to use chemical biological tools to investigate its biological effects is very attractive. A peptide comprising a TAT-conjugated Nrf2 sequence is shown to activate Nrf2 and its downstream target gene heme-oxygenase-1 (HO-1) in a dose-dependent manner in intact human THP-1 monocytes. Levels of Nrf2 protein peak after 3 h, whereas HO-1 mRNA and protein peak after 6 and 12 h, respectively. The peptide is also shown to inhibit the production of the pro-inflammatory cytokine TNF. The TAT-14mer constitutes a useful chemical biology tool with potential therapeutic applications.


