INNO-206Prodrug of doxorubicin CAS# 1361644-26-9 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1361644-26-9 | SDF | Download SDF |
| PubChem ID | 9810709 | Appearance | Powder |
| Formula | C37H42N4O13 | M.Wt | 750.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Aldoxorubicin; DOXO-EMCH | ||
| Solubility | DMSO : 75 mg/mL (99.90 mM; Need ultrasonic) | ||
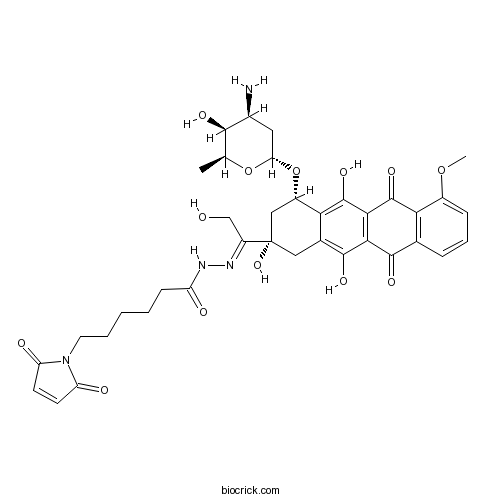
| Chemical Name | N-[(E)-[1-[(2S,4S)-4-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-3,4-dihydro-1H-tetracen-2-yl]-2-hydroxyethylidene]amino]-6-(2,5-dioxopyrrol-1-yl)hexanamide | ||
| SMILES | CC1C(C(CC(O1)OC2CC(CC3=C(C4=C(C(=C23)O)C(=O)C5=C(C4=O)C=CC=C5OC)O)(C(=NNC(=O)CCCCCN6C(=O)C=CC6=O)CO)O)N)O | ||
| Standard InChIKey | OBMJQRLIQQTJLR-USGQOSEYSA-N | ||
| Standard InChI | InChI=1S/C37H42N4O13/c1-17-32(46)20(38)13-27(53-17)54-22-15-37(51,23(16-42)39-40-24(43)9-4-3-5-12-41-25(44)10-11-26(41)45)14-19-29(22)36(50)31-30(34(19)48)33(47)18-7-6-8-21(52-2)28(18)35(31)49/h6-8,10-11,17,20,22,27,32,42,46,48,50-51H,3-5,9,12-16,38H2,1-2H3,(H,40,43)/b39-23+/t17-,20-,22-,27-,32+,37-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | INNO-206 is a prodrug of the anticancer agent doxorubicin, which is released from albumin under acidic conditions.In Vitro:INNO-206 (0.27 to 2.16 μM) inhibits blood vessel formation and reduces multiple myeloma cell growth in a pH-dependent fashion[1].In Vivo:INNO-206 (10.8 mg/kg, i.v.) shows significantly smaller tumor volumes and IgG levels on days 28, and is well tolerated with 90% of mice surviving until the termination of the study in the mice bearing the LAGκ-1A tumor[1]. INNO-206 shows a good safety profile at doses up to 260 mg/mL doxorubicin equivalents, and is able to induce tumor regressions in breast cancer, small cell lung cancer and sarcoma in phase I study[2]. INNO-206 shows superior activity over doxorubicin in a murine renal cell carcinoma model and in breast carcinoma xenograft models[3]. References: | |||||

INNO-206 Dilution Calculator

INNO-206 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.332 mL | 6.66 mL | 13.32 mL | 26.64 mL | 33.3 mL |
| 5 mM | 0.2664 mL | 1.332 mL | 2.664 mL | 5.328 mL | 6.66 mL |
| 10 mM | 0.1332 mL | 0.666 mL | 1.332 mL | 2.664 mL | 3.33 mL |
| 50 mM | 0.0266 mL | 0.1332 mL | 0.2664 mL | 0.5328 mL | 0.666 mL |
| 100 mM | 0.0133 mL | 0.0666 mL | 0.1332 mL | 0.2664 mL | 0.333 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
INNO-206 is an albumin-binding prodrug of doxorubicin [1].
Doxorubicin is one of the most active antitumor drugs and is used in the treatment of hematologic and solid cancers, such as breast and ovarian carcinoma, sarcoma and many other solid tumors [1].
INNO-206 is a prodrug of the anticancer drug doxorubicin, which is selectively bound to the Cys34 position of endogenous albumin after intravenous administration. In tumor cell lines, INNO-206 showed antitumor activity with IC50 values of ~0.4-1.1 μM in a 72-h cytotoxicity assay [1]. In multiple myeloma cell lines RPMI8226, U266, and MM1S, INNO-206 decreased in viable RPMI8226 cells in pH-dependent way. Also, it inhibited the cell growth in a concentration and pH-dependent way [2].
In LAGk-2-bearing mice, INNO-206 (1.8 mg/kg, 3 times weekly) significantly reduced tumor volume on days 28, 35, 42, 49, and 56, respectively.
References:
[1]. Kratz F. DOXO-EMCH (INNO-206): the first albumin-binding prodrug of doxorubicin to enter clinical trials. Expert Opin Investig Drugs, 2007, 16(6): 855-866.
[2]. Sanchez E, Li M, Wang C, et al. Anti-myeloma effects of the novel anthracycline derivative INNO-206. Clin Cancer Res, 2012, 18(14): 3856-3867.
- E3330
Catalog No.:BCC6421
CAS No.:136164-66-4
- (S)-(+)-Dimethindene maleate
Catalog No.:BCC7061
CAS No.:136152-65-3
- Minocycline HCl
Catalog No.:BCC4679
CAS No.:13614-98-7
- 3,7-Di-O-methylducheside A
Catalog No.:BCN6191
CAS No.:136133-08-9
- Przewalskinic acid A
Catalog No.:BCN2925
CAS No.:136112-75-9
- LDC1267
Catalog No.:BCC5577
CAS No.:1361030-48-9
- 14-Dehydrodelcosine
Catalog No.:BCN8119
CAS No.:1361-18-8
- Isomucronulatol 7-O-beta-glucoside
Catalog No.:BCN8088
CAS No.:136087-29-1
- Lobetyolin
Catalog No.:BCN5894
CAS No.:136085-37-5
- Onjixanthone II
Catalog No.:BCN7559
CAS No.:136083-93-7
- 5-Methoxyisolariciresinol
Catalog No.:BCN7016
CAS No.:136082-41-2
- Necrosulfonamide
Catalog No.:BCC7992
CAS No.:1360614-48-7
- Lobetyol
Catalog No.:BCN3321
CAS No.:136171-87-4
- 6-O-Caffeoylarbutin
Catalog No.:BCN6192
CAS No.:136172-60-6
- Sarranicine
Catalog No.:BCN2025
CAS No.:136173-25-6
- Neosarracine
Catalog No.:BCN2026
CAS No.:136173-26-7
- Neosarranicine
Catalog No.:BCN2024
CAS No.:136173-27-8
- PALDA
Catalog No.:BCC7287
CAS No.:136181-87-8
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- 3,4'-Dihydroxy-3',5'-dimethoxypropiophenone
Catalog No.:BCN1579
CAS No.:136196-47-9
- KW 3902
Catalog No.:BCC6124
CAS No.:136199-02-5
- Absinthiin
Catalog No.:BCN2314
CAS No.:1362-42-1
- GNE-617
Catalog No.:BCC4280
CAS No.:1362154-70-8
- TAT 14
Catalog No.:BCC6295
CAS No.:1362661-34-4
DOXO-EMCH (INNO-206): the first albumin-binding prodrug of doxorubicin to enter clinical trials.[Pubmed:17501697]
Expert Opin Investig Drugs. 2007 Jun;16(6):855-66.
The (6-maleimidocaproyl)hydrazone derivative of doxorubicin (DOXO-EMCH) is an albumin-binding prodrug of doxorubicin with acid-sensitive properties that demonstrates superior antitumor efficacy in murine tumor models and a favorable toxicity profile in mice, rats and dogs, including significantly reduced cardiotoxicity. After intravenous administration, DOXO-EMCH binds rapidly to the Cys-34 position of circulating albumin and accumulates in solid tumors due to passive targeting. In a clinical Phase I study, the dose of doxorubicin could be increased by a factor of 4.5-340 mg/m(2) when 75 mg/m(2) of free doxorubicin is considered to be the dose that can be administered as a single agent concomitant with the typical spectrum of side effects (i.e., myelotoxicity and mucositis). DOXO-EMCH was able to induce tumor regressions in anthracycline-sensitive tumors (i.e., breast cancer, small cell lung cancer and sarcoma). Phase II studies will be initiated at the beginning of 2007.
INNO-206, the (6-maleimidocaproyl hydrazone derivative of doxorubicin), shows superior antitumor efficacy compared to doxorubicin in different tumor xenograft models and in an orthotopic pancreas carcinoma model.[Pubmed:19148580]
Invest New Drugs. 2010 Feb;28(1):14-9.
The (6-maleimidocaproyl)hydrazone derivative of doxorubicin (INNO-206) is an albumin-binding prodrug of doxorubicin with acid-sensitive properties that is being assessed clinically. The prodrug binds rapidly to circulating serum albumin and releases doxorubicin selectively at the tumor site. This novel mechanism may provide enhanced antitumor activity of doxorubicin while improving the overall toxicity profile. Preclinically, INNO-206 has shown superior activity over doxorubicin in a murine renal cell carcinoma model and in breast carcinoma xenograft models. In this work, we compared the antitumor activity of INNO-206 and doxorubicin at their respective maximum tolerated doses in three additional xenograft models (breast carcinoma 3366, ovarian carcinoma A2780, and small cell lung cancer H209) as well as in an orthotopic pancreas carcinoma model (AsPC-1). INNO-206 showed more potent antitumor efficacy than free doxorubicin in all tumor models and is thus a promising clinical candidate for treating a broad range of solid tumors.
Anti-myeloma effects of the novel anthracycline derivative INNO-206.[Pubmed:22619306]
Clin Cancer Res. 2012 Jul 15;18(14):3856-67.
PURPOSE: Doxorubicin has shown efficacy especially in combination treatment for the treatment of multiple myeloma; however, its side effects limit its use. INNO-206 is an albumin-binding prodrug of doxorubicin, which is released from albumin under acidic conditions. Because INNO-206 has not been previously evaluated in any hematologic malignancy, we determined its anti-multiple myeloma effects. EXPERIMENTAL DESIGN: The anti-multiple myeloma effect of INNO-206 at different pH levels on multiple myeloma cell proliferation using multiple myeloma cell lines with the MTS assay and antiangiogenic activity using the chorioallantoic membrane/feather bud assay were determined. The anti-multiple myeloma effects and toxicity of INNO-206 were also compared with conventional doxorubicin and PEGylated liposomal doxorubicin (PLD) alone, and in combination with bortezomib, using our multiple myeloma xenograft models. RESULTS: INNO-206 inhibited blood vessel formation and reduced multiple myeloma cell growth in a pH-dependent fashion. INNO-206 alone produced marked anti-multiple myeloma effects in vivo at doses that doxorubicin was toxic, and the combination of INNO-206 plus bortezomib produced increased anti-multiple myeloma effects compared with either agent alone. In contrast, all mice receiving bortezomib with doxorubicin or PLD died. CONCLUSIONS: These findings show that INNO-206 produces anti-multiple myeloma effects in vitro and in vivo. It also enhances the antitumor effects of bortezomib. These results suggest that INNO-206 may provide patients with multiple myeloma with an anthracycline that may be administered safely at higher doses compared with free doxorubicin, resulting in superior efficacy compared with the currently available anthracyclines to treat this B-cell malignancy.


