Minocycline HClInhibitor of MMP activity CAS# 13614-98-7 |
Quality Control & MSDS
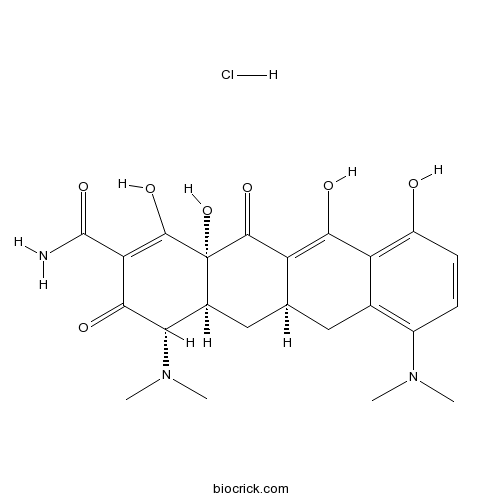
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13614-98-7 | SDF | Download SDF |
| PubChem ID | 54685925 | Appearance | Powder |
| Formula | C23H28ClN3O7 | M.Wt | 493.94 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 10 mg/mL (20.25 mM; Need ultrasonic) DMSO : 0.49 mg/mL (0.99 mM; Need ultrasonic and warming) | ||
| Chemical Name | (4S,4aS,5aR,12aR)-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide;hydrochloride | ||
| SMILES | CN(C)C1C2CC3CC4=C(C=CC(=C4C(=C3C(=O)C2(C(=C(C1=O)C(=O)N)O)O)O)O)N(C)C.Cl | ||
| Standard InChIKey | WTJXVDPDEQKTCV-VQAITOIOSA-N | ||
| Standard InChI | InChI=1S/C23H27N3O7.ClH/c1-25(2)12-5-6-13(27)15-10(12)7-9-8-11-17(26(3)4)19(29)16(22(24)32)21(31)23(11,33)20(30)14(9)18(15)28;/h5-6,9,11,17,27-28,31,33H,7-8H2,1-4H3,(H2,24,32);1H/t9-,11-,17-,23-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tetracycline antibiotic. Displays neuroprotective, antiapoptotic, anti-inflammatory and antimicrobial effects. Acts as a matrix metalloproteinase (MMP) inhibitor; attenuates disease severity in mouse models of multiple sclerosis. Orally active and brain penetrant. |

Minocycline HCl Dilution Calculator

Minocycline HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0245 mL | 10.1227 mL | 20.2454 mL | 40.4907 mL | 50.6134 mL |
| 5 mM | 0.4049 mL | 2.0245 mL | 4.0491 mL | 8.0981 mL | 10.1227 mL |
| 10 mM | 0.2025 mL | 1.0123 mL | 2.0245 mL | 4.0491 mL | 5.0613 mL |
| 50 mM | 0.0405 mL | 0.2025 mL | 0.4049 mL | 0.8098 mL | 1.0123 mL |
| 100 mM | 0.0202 mL | 0.1012 mL | 0.2025 mL | 0.4049 mL | 0.5061 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Minocycline is a tetracycline antibiotic with neuroprotective, antiapoptotic, anti-inflammatory and antimicrobial effects.
- 3,7-Di-O-methylducheside A
Catalog No.:BCN6191
CAS No.:136133-08-9
- Przewalskinic acid A
Catalog No.:BCN2925
CAS No.:136112-75-9
- LDC1267
Catalog No.:BCC5577
CAS No.:1361030-48-9
- 14-Dehydrodelcosine
Catalog No.:BCN8119
CAS No.:1361-18-8
- Isomucronulatol 7-O-beta-glucoside
Catalog No.:BCN8088
CAS No.:136087-29-1
- Lobetyolin
Catalog No.:BCN5894
CAS No.:136085-37-5
- Onjixanthone II
Catalog No.:BCN7559
CAS No.:136083-93-7
- 5-Methoxyisolariciresinol
Catalog No.:BCN7016
CAS No.:136082-41-2
- Necrosulfonamide
Catalog No.:BCC7992
CAS No.:1360614-48-7
- 9-Dehydroxyeurotinone
Catalog No.:BCN7397
CAS No.:1360606-85-4
- Desrhamnosylmartynoside
Catalog No.:BCN7648
CAS No.:136055-64-6
- [D-Lys3]-GHRP-6
Catalog No.:BCC5850
CAS No.:136054-22-3
- (S)-(+)-Dimethindene maleate
Catalog No.:BCC7061
CAS No.:136152-65-3
- E3330
Catalog No.:BCC6421
CAS No.:136164-66-4
- INNO-206
Catalog No.:BCC1651
CAS No.:1361644-26-9
- Lobetyol
Catalog No.:BCN3321
CAS No.:136171-87-4
- 6-O-Caffeoylarbutin
Catalog No.:BCN6192
CAS No.:136172-60-6
- Sarranicine
Catalog No.:BCN2025
CAS No.:136173-25-6
- Neosarracine
Catalog No.:BCN2026
CAS No.:136173-26-7
- Neosarranicine
Catalog No.:BCN2024
CAS No.:136173-27-8
- PALDA
Catalog No.:BCC7287
CAS No.:136181-87-8
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- 3,4'-Dihydroxy-3',5'-dimethoxypropiophenone
Catalog No.:BCN1579
CAS No.:136196-47-9
- KW 3902
Catalog No.:BCC6124
CAS No.:136199-02-5
A phase IV, open-label study evaluating the use of triple-combination therapy with minocycline HCl extended-release tablets, a topical antibiotic/retinoid preparation and benzoyl peroxide in patients with moderate to severe acne vulgaris.[Pubmed:23839176]
J Drugs Dermatol. 2013 Jun 1;12(6):619-25.
BACKGROUND: Moderate to severe acne vulgaris is often treated with a combination of an oral antibiotic, topical antibiotic/retinoid, and benzoyl peroxide (BP), but data are limited on the efficacy of this and other combination regimens that incorporate both oral and topical therapies.
METHODS: Patients were required to be aged 12-30 years with moderate to severe acne (grades 3-4 acne on the Investigator's Global Assessment [IGA]) and deemed potential candidates for treatment with isotretinoin. Enrolled patients were given triple-combination therapy, defined in this study as oral Minocycline HCl extended release 1 mg/kg QD, 6% BP foaming cloths used QD, and clindamycin phosphate 1.2%/tretinoin 0.025% gel applied QD, and were evaluated at baseline and weeks 2, 4, 8, and 12.
RESULTS: A total of 97 patients were enrolled in the study. At week 12, 89% of patients had at least a one-grade improvement from baseline IGA and 96% had at least a one-grade improvement from baseline Global Aesthetic Improvement Scale score. Mean +/- SD in- flammatory, non-inflammatory, and total lesion counts decreased from baseline by 61.8% +/- 38.3%, 48.8% +/- 34.5%, and 56.5% +/- 29.9%, respectively. The percentage of patients evaluated as candidates for isotretinoin by independent photographic review was 77% (69/90) at baseline and only 16% (14/90) at week 12. Treatment-related adverse events (AEs) occurred in eight of 97 (8%) patients. Triplecombination therapy was not associated with any serious AEs or AEs leading to discontinuation.
CONCLUSION: Triple-combination therapy was well tolerated and substantially reduced facial acne lesion counts, with 84% of patients judged to no longer be candidates for isotretinoin therapy by study end. These data support the clinical observation that a triple-combination regimen incorporating oral minocycline (dosed by patient weight), BP foaming cloths 6% QD, and clindamycin phosphate 1.2%/ tretinoin 0.025% gel QD can substantially improve moderate to severe acne vulgaris.
Comparative study to investigate the effect of meloxicam or minocycline HCl in situ gel system on local treatment of periodontal pockets.[Pubmed:24831089]
AAPS PharmSciTech. 2014 Aug;15(4):1021-8.
In situ gelling formulations allow easy application to the target area. Gelation is induced by physiological stimuli at the site of application where the formula attains semisolid properties and exerts sustained drug release. In situ gelling formulations containing either 3% meloxicam (Mx) or 2% Minocycline HCl (MH) were prepared for local application into the periodontal pockets. Gel formulations were based on the thermosensitive Pluronic((R)) (Pl) and the pH-sensitive Carbopol((R)) (C) polymers. C gels were prepared in combination with HPMC (H) to decrease its acidity. The total percent drug released from Pl formulae was 21.72% after 1 week for Mx and 85% after 3 days for MH. Their release kinetics data indicated anomalous non-Fickian behavior that could be controlled by both diffusion and chain relaxation. Addition of MH to C/H gels (1:2.5) resulted in liquefaction, followed by drug precipitation. Regarding C/H gel containing Mx, it showed a prolonged release rate up to 7 days with an initial burst effect; the kinetics data revealed Fickian-diffusion mechanism. The in vitro antibacterial activity studies for MH gel in Pl revealed that the drug released exceeded the minimum inhibitory concentration (MIC) of MH against Staphylococcus aureus ATCC 6538; placebo gel showed no effect on the microorganism. Clinical evaluation of Pl gels containing either Mx or MH showed significant improvement in chronic periodontitis patients, manifested by decrease in pocket depth and gingival index and increase in bone density.
The risk reduction of recurrent periodontal pathogens of local application minocycline HCl 2% gel, used as an adjunct to scaling and root planing for chronic periodontitis treatment.[Pubmed:28331333]
Ther Clin Risk Manag. 2017 Mar 10;13:307-314.
BACKGROUND: The aim of this study was to evaluate the clinical and microbiological effects of local application Minocycline HCl 2% gel, used as an adjunct to scaling and root planing (SRP) for treatment of chronic periodontitis (CP). CP is an inflammation of periodontal tissue that is caused mainly by bacterial infection, where periodontal destruction such as loss of attachment and bone destruction occurred. METHODS: A total of 81 subjects with moderate to severe periodontitis whose baseline clinical attachment loss (CAL) was >/=4 mm were randomly assigned to receive SRP alone (control group, N=39) or SRP followed by four times of local application of Minocycline HCl gel (Periocline) once a week (test group, N=42). Pocket depth, CAL, and papilla bleeding index were examined at baseline, 21 days, 2, 3, and 6 months. Subgingival plaque samples were collected with sterile curettes and were analyzed by real-time polymerase chain reaction for the presence of three periodontal pathogens (Porphyromonas gingivalis [P.g.], Tannerella forsythia [T.f.], and Treponema denticola [T.d.]) at baseline, 2, 3, and 6 months. RESULTS: The number of bacteria was reduced in both groups at 2 months after baseline (SRP treatment). The changes (2-6 months) in T.d. and T.f. counts in the test group were significantly lower than those in the control group. In the control group, a significant regrowth of P.g., T.f., and T.d. was observed from 2 to 6 months and of P.g. and T.f. from 3 to 6 months. On the other hand, in the test group, the number of the three bacteria did not significantly increase during the 6-month period. CONCLUSION: The results showed that local application of minocycline, used as an adjunct to SRP, was effective for suppressing regrowth of periodontal pathogens, suggesting its risk reduction of recurrent periodontal pathogens in CP.
Effects of minocycline-HCl paste root conditioning on periodontal surgery: in vitro and in vivo studies.[Pubmed:26064313]
Int J Clin Exp Med. 2015 Mar 15;8(3):4080-6. eCollection 2015.
OBJECTIVES: This study was aimed to investigate effects of 2% minocycline-HCl paste root conditioning on periodontal surgery. MATERIALS AND METHODS: In vitro, cementum slices affected by periodontitis were randomly conditioned with 2% minocycline-HCl paste, 2% minocycline-HCl liquid, and 0.9% saline. NIH3T3 cells were cultured and attached to the each slide, and the viability and proliferation of cells were observed; In vivo, 21 deep periodontal pockets were treated by periodontal surgery, and the exposed root surfaces were randomly conditioned with 2% minocycline-HCl paste or 0.9% saline. The periodontal parameters were measured at baseline, after 3 and 6 months of periodontal surgery. RESULTS: In vitro, NIH3T3 cell showed better viability and proliferation at 3, 5, and 7 day in groups conditioned with minocycline-HCl than the group conditioned with 0.9% saline (at 3 day (P < 0.05); at 7 day (P < 0.01)) Minimal differences were found between minocycline-HCl paste and liquid groups; In vivo, 3 months after periodontal surgery, the greater CAL reduction was found in the minocycline-HCl treated group than in the control group (P < 0.05). The similar results were found for both CAL and PD (P < 0.05; P < 0.05) between two groups at 6 months after surgery. PI and SBI variations showed no statistical differences between two groups after periodontal surgery. CONCLUSION: Our results suggested that root conditioning with minocycline-HCl paste during periodontal surgery improve the periodontal healing, which may be associated with the promotion of the periodontal cell attachment and growth onto the root surfaces.
Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms.[Pubmed:18952075]
Eur J Pharmacol. 2008 Dec 28;601(1-3):79-87.
Glia, particularly astrocytes and microglia, are known to play an important role in central sensitization and are strongly implicated in the exaggerated pain states. In the present study, we determined the effect of minocycline, an inhibitor of microglial activation, in acute nociception, peritonitis, and the development and maintenance of hypersensitivity following chronic constriction injury of the sciatic nerve in rats. A single dose of minocycline (30 or 100 mg/kg, i.p.) 30 min before acetic acid or zymosan injection did not attenuate the nociceptive behavior in mice. It had no effect on the early events of peritoneal inflammation (vascular permeability, inflammatory cell infiltration, and release of pro-inflammatory cytokines) in acetic acid or zymosan-injected mice. In addition, minocycline (30 or 100 mg/kg, i.p.) did not alter basal nociceptive responses in the tail immersion test. Chronic administration of minocycline (10 or 30 mg/kg, i.p.) for 7 days started before nerve injury significantly prevented the development of neuropathic pain, interestingly, it further delayed the development of hypersensitivity. In contrast, single injection of minocycline failed to reverse hypersensitivity when administered during the development of neuropathic pain. No significant effects were observed on hypersensitivity when treatment was started once neuropathic state was established. Pre-treatment, but not post-treatment, with minocycline markedly attenuated increased pro-inflammatory cytokines release and oxidative and nitrosative stress in mononeuropathic rats. These results suggest that minocycline had no effect on acute peritoneal inflammation, nociception, and chronic administration of minocycline when started early before peripheral nerve injury could attenuate and further delays the development of neuropathic pain. Concluding, this study clearly shows minocycline, an inhibitor of microglial activation, by inhibiting the release of pro-inflammatory mediators and reducing oxidative stress prevented the development of neuropathic pain.
Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis.[Pubmed:12023318]
Brain. 2002 Jun;125(Pt 6):1297-308.
Multiple sclerosis is characterized by the infiltration of leukocytes into the CNS. As matrix metalloproteinases (MMPs) facilitate the passage of leukocytes across matrix barriers, we tested the hypothesis that targeting MMPs could attenuate neuro-inflammation. We report that minocycline, a widely used generic drug with a good safety record, inhibited MMP activity, reduced production of MMP-9 and decreased the transmigration of T lymphocytes across a fibronectin matrix barrier. In addition, minocycline was efficacious against both mild and severe experimental autoimmune encephalomyelitis (EAE) in mice, an animal model of multiple sclerosis. When severe EAE was produced, minocycline pre-treatment delayed the course of the disease: when maximal disease activity occurred in vehicle-treated EAE mice, minocycline animals were relatively normal and had minimal signs of inflammation and demyelination in the CNS. When tested in mice afflicted with mild EAE, minocycline attenuated the clinical severity of disease throughout the course of treatment. These results indicate that minocycline may constitute a safe and inexpensive therapy for multiple sclerosis.
Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia.[Pubmed:11306611]
J Neurosci. 2001 Apr 15;21(8):2580-8.
Minocycline, a semisynthetic tetracycline derivative, protects brain against global and focal ischemia in rodents. We examined whether minocycline reduces excitotoxicity in primary neuronal cultures. Minocycline (0.02 microm) significantly increased neuronal survival in mixed spinal cord (SC) cultures treated with 500 microm glutamate or 100 microm kainate for 24 hr. Treatment with these excitotoxins induced a dose-dependent proliferation of microglia that was associated with increased release of interleukin-1beta (IL-1beta) and was followed by increased lactate dehydrogenase (LDH) release. The excitotoxicity was enhanced when microglial cells were cultured on top of SC cultures. Minocycline prevented excitotoxin-induced microglial proliferation and the increased release of nitric oxide (NO) metabolites and IL-1beta. Excitotoxins induced microglial proliferation and increased the release of NO metabolites and IL-1beta also in pure microglia cultures, and these responses were inhibited by minocycline. In both SC and pure microglia cultures, excitotoxins activated p38 mitogen-activated protein kinase (p38 MAPK) exclusively in microglia. Minocycline inhibited p38 MAPK activation in SC cultures, and treatment with SB203580, a p38 MAPK inhibitor, but not with PD98059, a p44/42 MAPK inhibitor, increased neuronal survival. In pure microglia cultures, glutamate induced transient activation of p38 MAPK, and this was inhibited by minocycline. These findings indicate that the proliferation and activation of microglia contributes to excitotoxicity, which is inhibited by minocycline, an antibiotic used in severe human infections.


