ABT-199Bcl-2 inhibitor,potent and selective CAS# 1257044-40-8 |
- Sabutoclax
Catalog No.:BCC2236
CAS No.:1228108-65-3
- HA14-1
Catalog No.:BCC3593
CAS No.:65673-63-4
- Obatoclax mesylate (GX15-070)
Catalog No.:BCC2234
CAS No.:803712-79-0
- TW-37
Catalog No.:BCC2257
CAS No.:877877-35-5
- Apogossypolone (ApoG2)
Catalog No.:BCC2237
CAS No.:886578-07-0
- ABT-263 (Navitoclax)
Catalog No.:BCC1272
CAS No.:923564-51-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1257044-40-8 | SDF | Download SDF |
| PubChem ID | 49846579 | Appearance | Powder |
| Formula | C45H50ClN7O7S | M.Wt | 868.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GDC-0199; Venetoclax | ||
| Solubility | Soluble to 100 mg/mL warmed (115.14 mM) in DMSO | ||
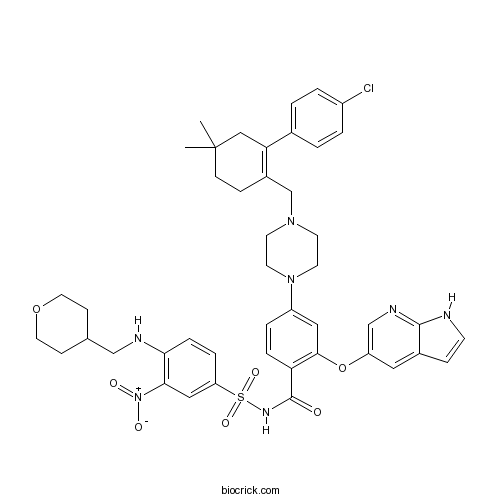
| Chemical Name | 4-[4-[[2-(4-chlorophenyl)-4,4-dimethylcyclohexen-1-yl]methyl]piperazin-1-yl]-N-[3-nitro-4-(oxan-4-ylmethylamino)phenyl]sulfonyl-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide | ||
| SMILES | CC1(CCC(=C(C1)C2=CC=C(C=C2)Cl)CN3CCN(CC3)C4=CC(=C(C=C4)C(=O)NS(=O)(=O)C5=CC(=C(C=C5)NCC6CCOCC6)[N+](=O)[O-])OC7=CN=C8C(=C7)C=CN8)C | ||
| Standard InChIKey | LQBVNQSMGBZMKD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | ABT-199 (GDC-0199) is a Bcl-2-selective inhibitor with Ki of <0.01 nM, >4800-fold more selective versus Bcl-xL and Bcl-w, and no activity to Mcl-1. | |||||
| Targets | Bcl-2 | |||||
| IC50 | < 0.010 nM (Ki) | |||||
| Cell experiment [1]: | |
| Cell lines | normal human B cells, as well as CD4+and CD8+ T cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 24h ;4 μM |
| Applications | We first determined the in vitro sensitivity to ABT-199 of normal human B cells, as well as CD4+and CD8+ T cells in peripheral blood sampled from healthy donors (n=9). Significantly, normal peripheral B cells were intrinsically more sensitive (~1000-fold) to ABT-199 than either T-cell subset (mean ABT-199 LC50±s.e.m. for B cells, CD4 T cells and CD8 T cells were 3.0 ±0.9 nM , 2.5±0.6 μM and 1.3±0.7 μM , respectively; B versus CD4 T cells: P=0.008; and B versus CD8 T cells: P=0.004). The result shown that normal human peripheral blood B cells are highly sensitive to ABT-199, unlike T cells and myeloid cells. |
| Animal experiment [1]: | |
| Animal models | Eμ-Myc mice |
| Dosage form | 100 mg/kg ; Oral taken |
| Application | We examined the effect of short-term treatment with ABT-199 (used at 100 mg/kg) on the lymphoid subpopulations in vivo to assess this and to model probable changes during therapy of patients. ABT-199 was administered orally, Consistent with our in vitro observations with murine and human cells, the drug substantially reduced peripheral B cells to a similar extent. These data suggested that because of intrinsic insensitivity to selective Bcl-2 inhibition of key B- and T-precursor cells, longer-term administration of ABT-199 may have an impact on normal lymphopoiesis to a lesser degree. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Khaw S L, Mérino D, Anderson M A, et al. Both leukaemic and normal peripheral B lymphoid cells are highly sensitive to the selective pharmacological inhibition of prosurvival Bcl-2 with ABT-199[J]. Leukemia, 2014, 28(6): 1207-1215. | |

ABT-199 Dilution Calculator

ABT-199 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1515 mL | 5.7575 mL | 11.5149 mL | 23.0298 mL | 28.7873 mL |
| 5 mM | 0.2303 mL | 1.1515 mL | 2.303 mL | 4.606 mL | 5.7575 mL |
| 10 mM | 0.1151 mL | 0.5757 mL | 1.1515 mL | 2.303 mL | 2.8787 mL |
| 50 mM | 0.023 mL | 0.1151 mL | 0.2303 mL | 0.4606 mL | 0.5757 mL |
| 100 mM | 0.0115 mL | 0.0576 mL | 0.1151 mL | 0.2303 mL | 0.2879 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ABT-199, developed through a structure-based reverse engineering process, is a novel and specific inhibitor of B-cell lymphoma/leukemia 2 (BCL-2) maintaining a sub-nanomolar affinity towards BCL-2 and over three orders of magnitude less affinity towards BCL-XL. It kills a diverse array of non-Hodgkin lymphoma (NHL) and acute myelogenous leukemia cell lines as well as BCL-2 dependent but not BCL-XL dependent cells via suppressing mitochondrial pathway of apoptosis, exhibiting potent antitumor activity against a wide variety of hematologic malignancies while sparing platelets. According to previous studies, ABT-199 is capable of suppressing tumor growth in several human hematologic tumor xenograft models.
Reference
Matthew S. Davids and Anthony Letai. ABT-199: a new hope for selective BCL-2 inhibition. Cancer Cell 2013; 23(2): 139-141
- TPTU
Catalog No.:BCC2827
CAS No.:125700-71-2
- TDBTU
Catalog No.:BCC2825
CAS No.:125700-69-8
- TBTU
Catalog No.:BCC2823
CAS No.:125700-67-6
- (-)-Epicatechin gallate
Catalog No.:BCN6327
CAS No.:1257-08-5
- Lavendustin A
Catalog No.:BCN1808
CAS No.:125697-92-9
- Lavendustin B
Catalog No.:BCN1809
CAS No.:125697-91-8
- CDK4 inhibitor
Catalog No.:BCC4242
CAS No.:1256963-02-6
- 2''-O-acetyl-platyconic acid A
Catalog No.:BCN3318
CAS No.:1256935-30-4
- 3''-O-acetyl-platyconic acid A
Catalog No.:BCN3319
CAS No.:1256935-28-0
- Blinin
Catalog No.:BCN8455
CAS No.:125675-09-4
- CH5424802
Catalog No.:BCC3749
CAS No.:1256580-46-7
- Ledipasvir
Catalog No.:BCC1696
CAS No.:1256388-51-8
- TC-SP 14
Catalog No.:BCC7926
CAS No.:1257093-40-5
- TC-G 24
Catalog No.:BCC6146
CAS No.:1257256-44-2
- NPEC-caged-noradrenalin
Catalog No.:BCC7835
CAS No.:1257323-83-3
- NPEC-caged-(S)-AMPA
Catalog No.:BCC7789
CAS No.:1257323-84-4
- NPEC-caged-(S)-3,4-DCPG
Catalog No.:BCC7652
CAS No.:1257323-85-5
- NPEC-caged-serotonin
Catalog No.:BCC7836
CAS No.:1257326-22-9
- NPEC-caged-dopamine
Catalog No.:BCC7837
CAS No.:1257326-23-0
- GSK 789472 hydrochloride
Catalog No.:BCC7818
CAS No.:1257326-24-1
- TC-E 5006
Catalog No.:BCC7981
CAS No.:1257395-14-4
- TBA354
Catalog No.:BCC6459
CAS No.:1257426-19-9
- Amikacin hydrate
Catalog No.:BCC4621
CAS No.:1257517-67-1
- CEP-33779
Catalog No.:BCC2199
CAS No.:1257704-57-6
Synergistic anti-leukemic interactions between ABT-199 and panobinostat in acute myeloid leukemia ex vivo.[Pubmed:27725868]
Am J Transl Res. 2016 Sep 15;8(9):3893-3902. eCollection 2016.
Cure rates for acute myeloid leukemia (AML) remain suboptimal; thus new treatment strategies are needed for this deadly disease. Poor clinical outcomes have been associated with overexpression of the anti-apoptotic Bcl-2 family proteins Bcl-2, Bcl-xL, and Mcl-1, which have garnered great interest as therapeutic targets. While the Bcl-2-selective inhibitor ABT-199 has demonstrated promising preclinical anti-leukemic activities, intrinsic drug resistance remains a problem. In our most recent study, we identified Mcl-1 sequestration of Bim as a mechanism of intrinsic resistance to ABT-199 in AML cells, thus upregulating Bim could overcome such resistance. Histone deacetylase (HDAC) inhibitors (HDACI) are a class of agents that have been confirmed to upregulate Bim. This prompted our hypothesis that combining an HDACI with ABT-199 would overcome intrinsic resistance to ABT-199 and result in synergistic anti-leukemic activity against AML. In this study, we investigated the anti-leukemic activity of panobinostat, a pan-HDACI, in combination with ABT-199 in AML cell lines and primary patient samples. We found that the combined drug treatment resulted in synergistic induction of cell death in both AML cell lines and primary patient samples. Panobinostat treatment resulted in upregulation of Bim, which remained elevated in the presence of ABT-199. In addition, shRNA knockdown of Bim in AML cell lines significantly attenuated apoptosis induced by combined panobinostat and ABT-199. Our results provide compelling evidence that Bim plays a key role in the combined anti-leukemic activity of panobinostat and ABT-199 against AML, and support clinical evaluation of combined panobinostat and ABT-199 in the treatment of AML.
The potential of venetoclax (ABT-199) in chronic lymphocytic leukemia.[Pubmed:27695617]
Ther Adv Hematol. 2016 Oct;7(5):270-287.
Venetoclax (VEN, ABT-199/GDC-0199) is an orally bioavailable BH3-mimetic that specifically inhibits the anti-apoptotic B-cell lymphoma/leukemia 2 (BCL2) protein. Although BCL2 overexpression is not genetically driven in chronic lymphocytic leukemia (CLL), it is nearly universal and represents a highly important and prevalent mechanism of apoptosis evasion, making it an attractive therapeutic target. This review summarizes the role of BCL2 in CLL pathogenesis, the development path targeting its inhibition prior to VEN, and the preclinical and clinical data regarding the effectiveness and safety of VEN. We further strive to contextualize VEN in the current CLL treatment landscape and discuss potential mechanisms of resistance.
Antitumor action of CDK inhibitor LS-007 as a single agent and in combination with ABT-199 against human acute leukemia cells.[Pubmed:27569395]
Acta Pharmacol Sin. 2016 Nov;37(11):1481-1489.
AIM: LS-007 is a CDK inhibitor, which exhibits potent antitumor activity against chronic lymphocytic leukemia and ovarian cancer cells. In this study, we further evaluated the antitumor activity of LS-007 alone and in combination with a Bcl-2 inhibitor ABT-199 in acute leukemia (AL) cells. METHODS: Cell viability was detected using resazurin assay, and cell apoptosis was examined using Annexin V/PI double staining and flow cytometry. The inhibition of LS-007 on kinases was evaluated with the mobility shift assay or ELISA. The expression of relevant signaling molecules was assessed using Western blotting and RT-PCR. Primary lymphocytes from patients with acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) were separated using Ficoll-Paque PLUS. RESULTS: LS-007 inhibited the proliferation of 6 AL cell lines with IC50 values of 100-200 nmol/L, and decreased the survival of ALL and AML patient-derived lymphocytes with mean LD50 value of 67 and 102 nmol/L, respectively. In kinase assays in vitro, LS-007 was more selective for the CDK family, inhibiting CDK2, CDK9, CDK1 and CDK4 at low nanomolar concentrations. In HL-60 and CCRF-CEM cells, LS-007 (0.1-0.4 mumol/L) dose-dependently induced cell apoptosis predominantly through CDK9 inhibition-related dephosphorylation at the ser2 residue of RNA pol II and the corresponding depletion of anti-apoptotic proteins, especially Mcl-1 and XIAP. LS-007 (0.2 and 0.4 mumol/L) also induced cell apoptosis in the patient-derived lymphocytes. In HL-60, CCRF-CEM and Molt-4 cells, combined application of LS-007 with ABT-199 (1 or 2 mumol/L) markedly increased cell apoptosis with a maximal decrease in the XIAP levels as compared with either drug used alone. CONCLUSION: CDK inhibitor LS-007 potently inhibits the established human AL cell lines and primary AL blasts, and it also shows remarkable synergy with Bcl-2 inhibitor ABT-199.
Duvelisib treatment is associated with altered expression of apoptotic regulators that helps in sensitization of chronic lymphocytic leukemia cells to venetoclax (ABT-199).[Pubmed:28017967]
Leukemia. 2017 Sep;31(9):1872-1881.
Duvelisib, an oral dual inhibitor of PI3K-delta and PI3K-gamma, is in phase III trials for the treatment of chronic lymphocytic leukemia (CLL) and indolent non-Hodgkin's lymphoma. In CLL, duvelisib monotherapy is associated with high iwCLL (International Workshop on Chronic Lymphocytic Leukemia) and nodal response rates, but complete remissions are rare. To characterize the molecular effect of duvelisib, we obtained samples from CLL patients on the duvelisib phase I trial. Gene expression studies (RNAseq, Nanostring, Affymetrix array and real-time RT-PCR) demonstrated increased expression of BCL2 along with several BH3-only pro-apoptotic genes. In concert with induction of transcript levels, reverse phase protein arrays and immunoblots confirmed increase at the protein level. The BCL2 inhibitor venetoclax induced greater apoptosis in ex vivo-cultured CLL cells obtained from patients on duvelisib compared with pre-treatment CLL cells from the same patients. In vitro combination of duvelisib and venetoclax resulted in enhanced apoptosis even in CLL cells cultured under conditions that simulate the tumor microenvironment. These data provide a mechanistic rationale for testing the combination of duvelisib and venetoclax in the clinic. Such combination regimen (NCT02640833) is being evaluated for patients with B-cell malignancies including CLL.


