Obatoclax mesylate (GX15-070)Potent Bcl-2 inhibitor CAS# 803712-79-0 |
- Marinopyrrole A
Catalog No.:BCC4098
CAS No.:1227962-62-0
- BM-1074
Catalog No.:BCC2235
CAS No.:1391108-10-3
- HA14-1
Catalog No.:BCC3593
CAS No.:65673-63-4
- ABT-737
Catalog No.:BCC3613
CAS No.:852808-04-9
- TW-37
Catalog No.:BCC2257
CAS No.:877877-35-5
- ABT-263 (Navitoclax)
Catalog No.:BCC1272
CAS No.:923564-51-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 803712-79-0 | SDF | Download SDF |
| PubChem ID | 16681698 | Appearance | Powder |
| Formula | C21H23N3O4S | M.Wt | 413.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Obatoclax Mesylate; GX15-070 | ||
| Solubility | DMSO : 12.5 mg/mL (30.23 mM; Need ultrasonic) | ||
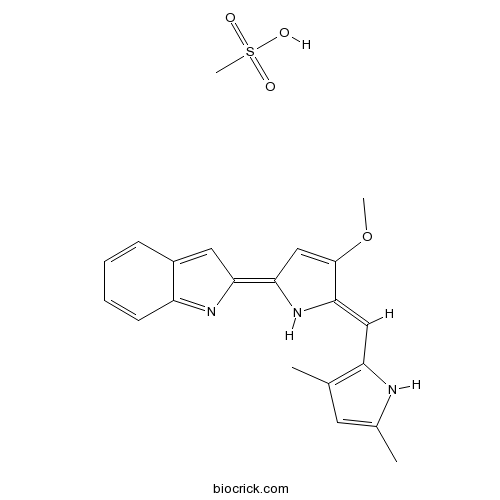
| Chemical Name | (2Z)-2-[(5Z)-5-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-4-methoxypyrrol-2-ylidene]indole;methanesulfonic acid | ||
| SMILES | CC1=CC(=C(N1)C=C2C(=CC(=C3C=C4C=CC=CC4=N3)N2)OC)C.CS(=O)(=O)O | ||
| Standard InChIKey | ZVAGBRFUYHSUHA-LZOXOEDVSA-N | ||
| Standard InChI | InChI=1S/C20H19N3O.CH4O3S/c1-12-8-13(2)21-16(12)10-19-20(24-3)11-18(23-19)17-9-14-6-4-5-7-15(14)22-17;1-5(2,3)4/h4-11,21,23H,1-3H3;1H3,(H,2,3,4)/b18-17-,19-10-; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Obatoclax (GX15-070) is an antagonist of Bcl-2 with Ki of 0.22 μM, can assist in overcoming MCL-1 mediated resistance to apoptosis. | |||||
| Targets | Bcl-2 | |||||
| IC50 | 0.22 μM (Ki) | |||||
| Cell experiment: [1] | |
| Cell lines | UMSCC-22A cells stably expressing GFP-LC3 |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 200 nM, 48 hours |
| Applications | After the treatment, cells were fixed in 4% paraformaldehyde and then stained with Hoechst 33258. A confocal microscope was used to visualize GFP-LC3 punctate dots. Treatment of these cells for 24 or 48 h with obatoclax (100 or 200 nM) resulted in relocalization of the GFP-LC3 protein to punctate cytoplasmic dots, an indicator of autophagosome formation. Treatment with obatoclax resulted in an approximately 10-fold increase in the average number of puncta per cell at 48 h as well as 24 h. |
| nimal experiment: [2] | |
| Animal models | Beige-nude-XID mice injected with SUDHL4 cells |
| Dosage form | Intraperitoneal injection, 3.0 mg/kg |
| Application | Obatoclax (3.0 mg/kg) had little effect on tumor growth while carfilzomib (2.0 mg/kg) by itself significantly reduced tumor size. Combined treatment resulted in minimal tumor growth, an effect significantly greater than that observed with either agent alone. IVIS imaging of luciferase-expressing tumor cells confirmed the marked reduction in tumor growth with combined therapy. Kaplan-Meier analysis also demonstrated that that carfilzomib significantly increased the survival of obatoclax-treated mice. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Yazbeck VY, Li C, Grandis JR, Zang Y, Johnson DE. Single-agent obatoclax (GX15-070) potently induces apoptosis and pro-survival autophagy in head and neck squamous cell carcinoma cells. Oral Oncol. 2014 Feb;50(2):120-7. [2] Dasmahapatra G, Lembersky D, Son MP, Patel H, Peterson D, Attkisson E, Fisher RI, Friedberg JW, Dent P, Grant S. Obatoclax interacts synergistically with the irreversible proteasome inhibitor carfilzomib in GC- and ABC-DLBCL cells in vitro and in vivo. Mol Cancer Ther. 2012 May;11(5):1122-32. | |

Obatoclax mesylate (GX15-070) Dilution Calculator

Obatoclax mesylate (GX15-070) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4184 mL | 12.0919 mL | 24.1838 mL | 48.3676 mL | 60.4595 mL |
| 5 mM | 0.4837 mL | 2.4184 mL | 4.8368 mL | 9.6735 mL | 12.0919 mL |
| 10 mM | 0.2418 mL | 1.2092 mL | 2.4184 mL | 4.8368 mL | 6.0459 mL |
| 50 mM | 0.0484 mL | 0.2418 mL | 0.4837 mL | 0.9674 mL | 1.2092 mL |
| 100 mM | 0.0242 mL | 0.1209 mL | 0.2418 mL | 0.4837 mL | 0.6046 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Obatoclax mesylate, also known as GX15-070, is a hydrophobic small molecule that potently inhibits BCL-2 family by binding to the BH3-binding site of BCL-2 and other related BCL-2 family members (including BCL-XL, MCL-1, A1, and BCL-B). As a pan-BCL-2 inhibitor being investigated for the treatment of refractory malignancies, obatoclax mesylate directly induce apoptosis in cultured acute myeloid leukemia (AML) cells as well as primary patient samples and exhibits antitumor activity in mouse xengografts of solid tumor and myeloma cell lines. Study results have shown that obatoclax mesylate inhibited clonogenic growth of primary AML samples (IC50 < nmol/L) and dissociated Bak and Bim from MCL-1 in cultured AML cells.
Reference
Aaron D. Schimmer, Susan O’Brien, Hagop Kantarjian, Joseph Brandwein, Bruce D. Cheson, Mark D. Minden, Karen Yee, Farhad Ravandi, Francis Giles, Andre Schuh, Vikas Gupta, Michael Andreeff, Charles Koller, Hong Chang, Suzanne Kamel-Reid, Mark Berger, Jean Viallet, and Gautam Borthakur. A phase I study of the Pan BCL-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res 2008; 14:8295-8301
- 8beta-Tigloyloxyreynosin
Catalog No.:BCN7222
CAS No.:80368-31-6
- 2',3,5,6',7-Pentahydroxyflavanone
Catalog No.:BCN4337
CAS No.:80366-15-0
- NSC59984
Catalog No.:BCC6540
CAS No.:803647-40-7
- Tinnevellin glucoside
Catalog No.:BCN3414
CAS No.:80358-06-1
- 8-Acetonyldihydronitidine
Catalog No.:BCN3304
CAS No.:80330-39-8
- Gypenoside XVII
Catalog No.:BCN2339
CAS No.:80321-69-3
- Stevenleaf
Catalog No.:BCN5978
CAS No.:80321-63-7
- Dehydro-δ-tocopherol
Catalog No.:BCN4573
CAS No.:802909-72-4
- RG7090
Catalog No.:BCC5499
CAS No.:802906-73-6
- AZD1981
Catalog No.:BCC4506
CAS No.:802904-66-1
- Artemisinic acid
Catalog No.:BCN4336
CAS No.:80286-58-4
- PHA-848125
Catalog No.:BCC3839
CAS No.:802539-81-7
- Z-D-Thr-OH
Catalog No.:BCC2736
CAS No.:80384-27-6
- Protoplumericin A
Catalog No.:BCN4572
CAS No.:80396-57-2
- 7-Acetylscorpioidine
Catalog No.:BCN2028
CAS No.:80405-17-0
- Scorpioidine
Catalog No.:BCN2027
CAS No.:80405-18-1
- 13-O-p-Coumaroylplumieride
Catalog No.:BCN4338
CAS No.:80416-52-0
- Notoginsenoside R1
Catalog No.:BCN1097
CAS No.:80418-24-2
- Notoginsenoside R2
Catalog No.:BCN3328
CAS No.:80418-25-3
- Calcium Levofolinate
Catalog No.:BCC4643
CAS No.:80433-71-2
- gamma-Diasarone
Catalog No.:BCN4339
CAS No.:80434-33-9
- Dynorphin A
Catalog No.:BCC7596
CAS No.:80448-90-4
- Padmatin
Catalog No.:BCN4340
CAS No.:80453-44-7
- Paeoniflorigenone
Catalog No.:BCN3933
CAS No.:80454-42-8
Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia.[Pubmed:18931344]
Blood. 2009 Jan 8;113(2):299-305.
Obatoclax mesylate is a small molecule pan-Bcl-2 antagonist with in vitro activity against chronic lymphocytic leukemia (CLL) cells. Obatoclax was administered to patients with advanced CLL at doses ranging from 3.5 to 14 mg/m(2) as a 1-hour infusion and from 20 to 40 mg/m(2) as a 3-hour infusion every 3 weeks. Twenty-six patients received a total of 74 cycles. Dose-limiting reactions were neurologic (somnolence, euphoria, ataxia) and associated with the infusion. The maximum tolerated dose (MTD) was 28 mg/m(2) over 3 hours every 3 weeks. One (4%) of 26 patients achieved a partial response. Patients with anemia (3/11) or thrombocytopenia (4/14) experienced improvements in hemoglobin and platelet counts. Circulating lymphocyte counts were reduced in 18 of 26 patients with a median reduction of 24%. Overall, the maximum plasma concentration (C(max)) and area under the curve (AUC) values of obatoclax were dose proportional. Activation of Bax and Bak was demonstrated in peripheral blood mononuclear cells, and induction of apoptosis was related to overall obatoclax exposure, as monitored by the plasma concentration of oligonucleosomal DNA/histone complexes. Obatoclax mesylate has biologic activity and modest single-agent activity in heavily pretreated patients with advanced CLL. Further evaluation in less heavily pretreated patients and in combination with other therapeutic agents is warranted. This trial has been registered with http://clinicaltrials.gov under identifier NCT00600964.
Phase II study of obatoclax mesylate (GX15-070), a small-molecule BCL-2 family antagonist, for patients with myelofibrosis.[Pubmed:20709666]
Clin Lymphoma Myeloma Leuk. 2010 Aug;10(4):285-9.
BACKGROUND: Myelofibrosis (MF) is a disease characterized by the overexpression of the antiapoptotic BCL-2 family of proteins (eg, BCL-XL and MCL-1). PATIENTS AND METHODS: We conducted a multicenter, open-label, noncomparative phase II study of obatoclax mesylate, a small-molecule pan-BCL-2 antagonist, in patients with MF. Obatoclax was administered as a 24-hour infusion (on an outpatient basis) every 2 weeks at a fixed dose of 60 mg. RESULTS: A total of 22 patients were enrolled, with a median age of 63 years (range, 43-89 years). Twelve were men, and all 22 patients were previously treated (median of 2 previous therapies). Ten patients (45%) had a Lille score of 1, and 9 patients (41%) had a Lille score of 2. Thirteen (59%) were red blood cell transfusion dependent. A median of 7 cycles of obatoclax were administered. No patient achieved complete or partial response according to International Working Group criteria. One patient (4%) demonstrated a clinical improvement (in terms of hemoglobin and platelet count) after 7 cycles of therapy. The improvement was sustained for 4 cycles of therapy, after which he underwent allogeneic stem cell transplantation. The most common adverse events included low-grade ataxia and fatigue in 50% of the patients. Dose reduction because of toxicity was required in 1 patient, whereas 2 patients were taken off the study because of grade 3 ataxia and grade 3 heart failure. Grade 3/4 anemia and thrombocytopenia were evident in 6 (27%) and 4 (18%) patients, respectively. CONCLUSION: Obatoclax exhibits no significant clinical activity in patients with MF at the dose and schedule evaluated.


