StevenleafCAS# 80321-63-7 |
- Pioglitazone HCl
Catalog No.:BCC2278
CAS No.:112529-15-4
- Rosiglitazone
Catalog No.:BCC2264
CAS No.:122320-73-4
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- T0070907
Catalog No.:BCC2261
CAS No.:313516-66-4
- Troglitazone
Catalog No.:BCC2016
CAS No.:97322-87-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 80321-63-7 | SDF | Download SDF |
| PubChem ID | 46887681 | Appearance | White powder |
| Formula | C47H80O17 | M.Wt | 917.2 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Ginsenoside C-Mx1; Gynosaponin I; Gypenoside IX; Notoginsenoside Fd | ||
| Solubility | DMSO : 25 mg/mL (27.26 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
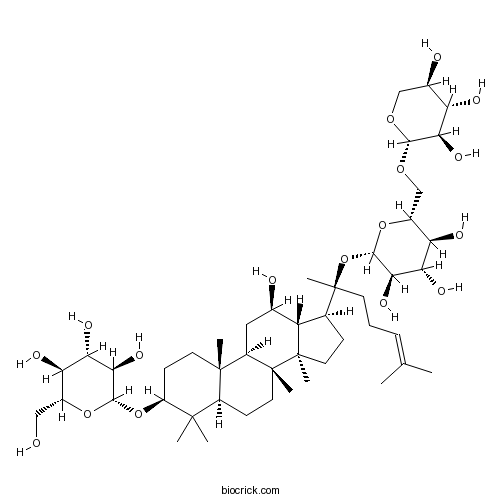
| Chemical Name | (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-[[(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-4,4,8,10,14-pentamethyl-17-[(2S)-6-methyl-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxymethyl]oxan-2-yl]oxyhept-5-en-2-yl]-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]oxane-3,4,5-triol | ||
| SMILES | CC(=CCCC(C)(C1CCC2(C1C(CC3C2(CCC4C3(CCC(C4(C)C)OC5C(C(C(C(O5)CO)O)O)O)C)C)O)C)OC6C(C(C(C(O6)COC7C(C(C(CO7)O)O)O)O)O)O)C | ||
| Standard InChIKey | ZTQSADJAYQOCDD-HUGMCNGHSA-N | ||
| Standard InChI | InChI=1S/C47H80O17/c1-22(2)10-9-14-47(8,64-42-39(58)36(55)34(53)27(62-42)21-60-40-37(56)32(51)25(50)20-59-40)23-11-16-46(7)31(23)24(49)18-29-44(5)15-13-30(43(3,4)28(44)12-17-45(29,46)6)63-41-38(57)35(54)33(52)26(19-48)61-41/h10,23-42,48-58H,9,11-21H2,1-8H3/t23-,24+,25+,26+,27+,28-,29+,30-,31-,32-,33+,34+,35-,36-,37+,38+,39+,40-,41-,42-,44-,45+,46+,47-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Gypenosides (Gyp, Stevenleaf) induce apoptosis in human hepatoma cells through the up-regulation of Bax and Bak, and down-regulation of Bcl-2, release of mitochondrial cytochrome c and activation of caspase cascade. 2. Gypenosides induce ER stress and production of reactive oxygen species and Ca 2+ , change the ratio of Bcl-2 and Bax, followed by the dysfunction of mitochondria, cause cytochrome c release, activation of caspase-3 before leading to apoptosis, these results provide information towards an understanding of the mechanisms by which Gyp induces cell cycle arrest and apoptosis in human tongue cancer cells. 3. Gypenosides can inhibit invasion and migration of human tongue SCC4 cells by down-regulating proteins associated with these processes, resulting in reduced metastasis. 4. Gypenosides imply their remarkable preventative and therapeutic potential in treatment of neurological diseases involving glutamate and oxidative stress. 5. The extensive antioxidant effect of gypenosides may be valuable to the prevention and treatment of various diseases such as atherosclerosis, liver disease and inflammation. |
| Targets | Bcl-2/Bax | Caspase | NF-kB | COX | ERK | MMP(e.g.TIMP) | NADPH-oxidase | Calcium Channel |

Stevenleaf Dilution Calculator

Stevenleaf Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0903 mL | 5.4514 mL | 10.9027 mL | 21.8055 mL | 27.2569 mL |
| 5 mM | 0.2181 mL | 1.0903 mL | 2.1805 mL | 4.3611 mL | 5.4514 mL |
| 10 mM | 0.109 mL | 0.5451 mL | 1.0903 mL | 2.1805 mL | 2.7257 mL |
| 50 mM | 0.0218 mL | 0.109 mL | 0.2181 mL | 0.4361 mL | 0.5451 mL |
| 100 mM | 0.0109 mL | 0.0545 mL | 0.109 mL | 0.2181 mL | 0.2726 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Gynostemma Extract is a natural product.
- Dehydro-δ-tocopherol
Catalog No.:BCN4573
CAS No.:802909-72-4
- RG7090
Catalog No.:BCC5499
CAS No.:802906-73-6
- AZD1981
Catalog No.:BCC4506
CAS No.:802904-66-1
- Artemisinic acid
Catalog No.:BCN4336
CAS No.:80286-58-4
- PHA-848125
Catalog No.:BCC3839
CAS No.:802539-81-7
- Nafamostat hydrochloride
Catalog No.:BCC4188
CAS No.:80251-32-7
- Spiramycin
Catalog No.:BCC4724
CAS No.:8025-81-8
- Casanthranol
Catalog No.:BCC3746
CAS No.:8024-48-4
- 2'-O-Galloylquercitrin
Catalog No.:BCN8225
CAS No.:80229-08-9
- Rosmanol
Catalog No.:BCN8425
CAS No.:80225-53-2
- Glochidionionol C
Catalog No.:BCC2641
CAS No.:
- Roxithromycin
Catalog No.:BCC4842
CAS No.:80214-83-1
- Gypenoside XVII
Catalog No.:BCN2339
CAS No.:80321-69-3
- 8-Acetonyldihydronitidine
Catalog No.:BCN3304
CAS No.:80330-39-8
- Tinnevellin glucoside
Catalog No.:BCN3414
CAS No.:80358-06-1
- NSC59984
Catalog No.:BCC6540
CAS No.:803647-40-7
- 2',3,5,6',7-Pentahydroxyflavanone
Catalog No.:BCN4337
CAS No.:80366-15-0
- 8beta-Tigloyloxyreynosin
Catalog No.:BCN7222
CAS No.:80368-31-6
- Obatoclax mesylate (GX15-070)
Catalog No.:BCC2234
CAS No.:803712-79-0
- Z-D-Thr-OH
Catalog No.:BCC2736
CAS No.:80384-27-6
- Protoplumericin A
Catalog No.:BCN4572
CAS No.:80396-57-2
- 7-Acetylscorpioidine
Catalog No.:BCN2028
CAS No.:80405-17-0
- Scorpioidine
Catalog No.:BCN2027
CAS No.:80405-18-1
- 13-O-p-Coumaroylplumieride
Catalog No.:BCN4338
CAS No.:80416-52-0
Gypenosides protect primary cultures of rat cortical cells against oxidative neurotoxicity.[Pubmed:16806111]
Brain Res. 2006 Aug 2;1102(1):163-74.
Gypenosides (GPs) were tested for their ability to protect primary cultures of immature cortical cells against oxidative glutamate toxicity. In immature neural cells, glutamate cytotoxicity is known to be mediated by the inhibition of cystine uptake, leading to depletion of intracellular glutathione (GSH). The depletion of GSH impairs cellular antioxidant defenses resulting in oxidative stress and cell death. We found that pretreatment with GPs (100-400 microg/ml) significantly protected cells from glutamate-induced cell death. It was therefore of interest to investigate whether GPs protect cortical cells against glutamate-induced oxidative injury through preventing GSH depletion. Results show that GPs significantly up-regulated mRNAs encoding gamma-glutamylcysteine synthetase (gamma-GCS) and glutathione reductase (GR) and enhanced their activities for GSH synthesis as well as recycle. Furthermore, GPs lowered the consumption of GSH through decreased accumulation of intracellular peroxides, leading to an increase in the intracellular GSH content. GPs were also found to prevent lipid peroxidation and reduce the influx of Ca(2+) which routinely follows glutamate oxidative challenge. GPs treatment significantly blocked glutamate-induced decrease in levels of Bcl-2 and increase in Bax, leading to a decrease in glutamate-induced apoptosis. Thus, we conclude that GPs protect cortical cells by multiple antioxidative actions via enhancing intracellular GSH, suppressing glutamate-induced cytosolic Ca(2+) elevation and blocking glutamate-induced apoptosis. The novel role of GPs implies their remarkable preventative and therapeutic potential in treatment of neurological diseases involving glutamate and oxidative stress.
Protective effect of gypenosides against oxidative stress in phagocytes, vascular endothelial cells and liver microsomes.[Pubmed:7804367]
Cancer Biother. 1993 Fall;8(3):263-72.
The action of gypenosides (GP, saponins of Gynostemma pentaphyllum, a Chinese medicinal herb) as an antioxidant was studied using various models of oxidant stress in phagocytes, liver microsomes and vascular endothelial cells. The results show that GP decreased superoxide anion and hydrogen peroxide content in human neutrophils and diminished chemiluminescent oxidative burst triggered by zymosan in human monocytes and murine macrophages. An increase of lipid peroxidation induced by Fe2+/cysteine, ascorbate/NADPH or hydrogen peroxide in liver microsomes and vascular endothelial cells was inhibited by GP. It was also found that GP protected biomembranes from oxidative injury by reversing the decreased membrane fluidity of liver microsomes and mitochondria, increasing mitochondrial enzyme activity in vascular endothelial cells and decreasing intracellular lactate dehydrogenase leakage from these cells. The extensive antioxidant effect of GP may be valuable to the prevention and treatment of various diseases such as atherosclerosis, liver disease and inflammation.
Regulation of Bcl-2 family molecules and activation of caspase cascade involved in gypenosides-induced apoptosis in human hepatoma cells.[Pubmed:12065092]
Cancer Lett. 2002 Sep 26;183(2):169-78.
Herbal medicines are increasingly being utilized to treat a wide variety of disease processes. Gypenosides (Gyp) are triterpenoid saponins contained in an extract from Gynostemma pentaphyllum Makino and reported to induce apoptosis in human hepatoma cells. However, the molecular mechanism underlying the Gyp-induced apoptotic process is unclear. In this study, we found that Gyp induced apoptosis in human hepatoma Huh-7, Hep3B and HA22T cell lines as evidenced by morphological changes, 4',6'-diamidino-2-phenylindole staining and in situ terminal transferase-mediated dUTP-fluorescensin nick end-labeling assay. Our data demonstrated that Gyp-induced apoptotic cell death was accompanied by up-regulation of Bax, Bak and Bcl-X(L), and down-regulation of Bcl-2 and Bad, while it had no effect on the level of Bag-1 protein. Moreover, Gyp treatment caused the release of mitochondrial cytochrome c to cytosol and sequential activation of caspases, including caspase-1, -9 and -3, then leading to cleavage of poly-ADP-ribose polymerase. Furthermore, the Gyp-induced apoptosis was markedly blocked by the broad-spectrum caspase inhibitor, z-VAD-fmk. Taken together, these results suggest that treatment of human hepatoma cells with Gyp induced apoptosis through the up-regulation of Bax and Bak, and down-regulation of Bcl-2, release of mitochondrial cytochrome c and activation of caspase cascade.
Gypenosides inhibited invasion and migration of human tongue cancer SCC4 cells through down-regulation of NFkappaB and matrix metalloproteinase-9.[Pubmed:18507059]
Anticancer Res. 2008 Mar-Apr;28(2A):1093-9.
Gypenosides (Gyp), components of Gynostemma pentaphyllum Makino, were found to induce suppression of human tongue squamous cell carcinoma SCC4 cell growth and induce apoptosis in response to overexpression of reactive oxygen species, calcium (Ca(+2)) and to decrease mitochondrial membrane potential in vitro. In this study, the effect of Gyp on cell migration and invasion of human tongue SCC4 cells was examined. SCC4 cells treated in vitro with Gyp migrated and invaded less than cells treated with phosphate-buffered saline (PBS) as a control. Gyp inhibited migration and invasion by down-regulating the production of RAS, NFkappaB, COX2, ERK1/2 and MMP-9 relative to PBS only. These results show that Gyp inhibits invasion and migration of human tongue SCC4 cells by down-regulating proteins associated with these processes, resulting in reduced metastasis.
Gypenosides induced G0/G1 arrest via CHk2 and apoptosis through endoplasmic reticulum stress and mitochondria-dependent pathways in human tongue cancer SCC-4 cells.[Pubmed:18674953]
Oral Oncol. 2009 Mar;45(3):273-83.
Gypenosides (Gyp), a component of Gynostemma pentaphyllum Makino, was selected for examining the effects on the cell viability, cell cycle and induction of apoptosis in human tongue cancer SCC-4 cells. Gyp induced cytotoxicity (decreased the percentage of viable cells) in SCC-4 cells appeared to be associated with induction of cell cycle arrest (G0/G1 arrest), apoptotic cell death based on Gyp induced morphological changes and DNA fragmentation and increased the sub-G1 group in examined SCC-4 cells. The production of reactive oxygen species and Ca(2+) and the depolarization of mitochondrial membrane potential were observed, dose- and time-dependently, after treatment of SCC-4 cells with various concentrations of Gyp. Gyp inhibited the levels of the anti-apoptotic proteins Bcl-2 and Bcl-xl, but promoted the levels of the pro-apoptotic protein Bax. Western blotting showed the releases of cytochrome c and Endo G and both were also confirmed by confocal laser microscopic systems. The GADD153 moved to nuclei (nuclear translocation). In conclusion, Gyp induced ER stress and production of reactive oxygen species and Ca(2+), change the ratio of Bcl-2 and Bax, followed by the dysfunction of mitochondria, caused cytochrome c release, activation of caspase-3 before leading to apoptosis. These results provide information towards an understanding of the mechanisms by which Gyp induces cell cycle arrest and apoptosis in human tongue cancer cells.


