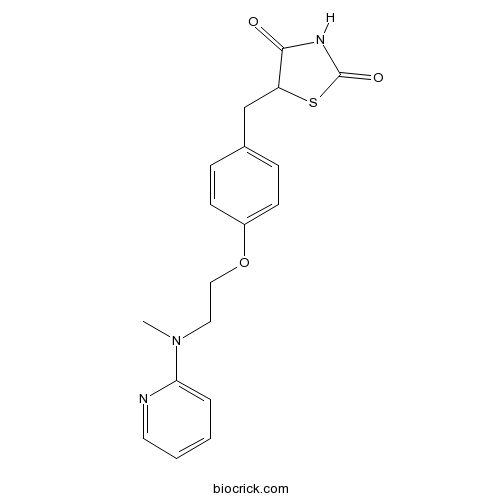
RosiglitazonePotent PPARγ agonist CAS# 122320-73-4 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- (R)-DRF053 dihydrochloride
Catalog No.:BCC7726
CAS No.:1241675-76-2
- Flavopiridol hydrochloride
Catalog No.:BCC3925
CAS No.:131740-09-5
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- PHA-848125
Catalog No.:BCC3839
CAS No.:802539-81-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 122320-73-4 | SDF | Download SDF |
| PubChem ID | 77999 | Appearance | Powder |
| Formula | C18H19N3O3S | M.Wt | 357.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BRL 49653 | ||
| Solubility | DMSO : ≥ 110 mg/mL (307.75 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 5-[[4-[2-[methyl(pyridin-2-yl)amino]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione | ||
| SMILES | CN(CCOC1=CC=C(C=C1)CC2C(=O)NC(=O)S2)C3=CC=CC=N3 | ||
| Standard InChIKey | YASAKCUCGLMORW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,15H,10-12H2,1H3,(H,20,22,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective PPARγ agonist (EC50 = 60 nM); exhibits no activity at PPARα and PPARβ. Promotes differentiation of pluripotent C3H10T1/2 stem cells into adipocytes. Exhibits antihyperglycemic activity in diabetic ob/ob mouse model. Antidiabetic agent. |

Rosiglitazone Dilution Calculator

Rosiglitazone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7978 mL | 13.9888 mL | 27.9775 mL | 55.955 mL | 69.9438 mL |
| 5 mM | 0.5596 mL | 2.7978 mL | 5.5955 mL | 11.191 mL | 13.9888 mL |
| 10 mM | 0.2798 mL | 1.3989 mL | 2.7978 mL | 5.5955 mL | 6.9944 mL |
| 50 mM | 0.056 mL | 0.2798 mL | 0.5596 mL | 1.1191 mL | 1.3989 mL |
| 100 mM | 0.028 mL | 0.1399 mL | 0.2798 mL | 0.5596 mL | 0.6994 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Rosiglitazone is a potent agonist of peroxisome proliferator-activated receptor γ (PPARγ), a subfamily of the nuclear-receptor superfamily which is predominately expressed in adipose tissue and regulates gene expression responding to ligand binding. Belonging to the thiazolidinedione (TZD) class, rosiglitazone, like other TZD members, binds to PPARγ DNA as heterodimers and activate transcription of various metabolic regulators involved in the differentiation of stem cells into adipocytes and increased expression of genes regulating the metabolism of glucose and lipid. Rosiglitazone is used to treat patients with type II diabetes mellitus for its strong ability to improve insulin sensitization through its effects either on fatty acid uptake and storage in adipose tissue or on adiokines.
Reference
Peter J. Cox, David A. Ryan, Frank J. Hollis, Ann-Marie Harris, Ann K. Miller, Marika Vousden and Hugh Cowley. Absorption, disposition, and metabolism of rosiglitazone, a potent thiazolidinedione insulin sensitizer, in humans. Drug Metabolism and Disposition 2000; 28(7): 772-780
Adie Vilioen and Alan Sinclair. Safety and efficacy of rosiglitazone in the elderly diabetic patient. Vascular Health and Risk Management 2009:5 389-395
- 6''-O-acetylisovitexin
Catalog No.:BCN6114
CAS No.:1223097-20-8
- Vermisporin
Catalog No.:BCN1863
CAS No.:122301-98-8
- QL-IX-55
Catalog No.:BCC1876
CAS No.:1223002-54-7
- Torin 2
Catalog No.:BCC4606
CAS No.:1223001-51-1
- Torin 1
Catalog No.:BCC3676
CAS No.:1222998-36-8
- ML324
Catalog No.:BCC5575
CAS No.:1222800-79-4
- ML133 HCl
Catalog No.:BCC5006
CAS No.:1222781-70-5
- 3-Epimeliasenin B
Catalog No.:BCN4723
CAS No.:1222475-77-5
- Boc-Dap(Fmoc)-OH
Catalog No.:BCC2665
CAS No.:122235-70-5
- 5'-Geranyl-5,7,2',4'-tetrahydroxyflavone
Catalog No.:BCN1601
CAS No.:1221762-70-4
- NPS-1034
Catalog No.:BCC6504
CAS No.:1221713-92-3
- Gymnemic acid I
Catalog No.:BCN2679
CAS No.:122168-40-5
- Amylin
Catalog No.:BCC6017
CAS No.:122384-88-7
- Paederosidic acid methyl ester
Catalog No.:BCN3439
CAS No.:122413-01-8
- MFZ 10-7
Catalog No.:BCC6180
CAS No.:1224431-15-5
- 4-O-(3-nitropropanoyl)corollin
Catalog No.:BCC8716
CAS No.:122475-42-7
- GBLD 345
Catalog No.:BCC6787
CAS No.:122479-08-7
- INK 128 (MLN0128)
Catalog No.:BCC3880
CAS No.:1224844-38-5
- Gentiside B
Catalog No.:BCN7301
CAS No.:1225022-67-2
- Naltriben mesylate
Catalog No.:BCC5683
CAS No.:122517-78-6
- AG 99
Catalog No.:BCC6667
CAS No.:122520-85-8
- DCC-2618
Catalog No.:BCC1520
CAS No.:1225278-16-9
- Gelomulide A
Catalog No.:BCN6580
CAS No.:122537-59-1
- Gelomulide B
Catalog No.:BCN6588
CAS No.:122537-60-4
PPARgamma agonist rosiglitazone protects rat peritoneal mesothelial cells against peritoneal dialysis solutioninduced damage.[Pubmed:28259952]
Mol Med Rep. 2017 Apr;15(4):1786-1792.
Long-term peritoneal dialysis (PD) leads to ultrafiltration failure (UFF). Peritoneal mesothelial cells, which form the innermost monolayer of the peritoneal cavity, have been shown to regulate various responses, including inflammation, in UFF. The present study was designed to investigate the effect of the peroxisome proliferatoractivated receptorgamma (PPARgamma) agonist, Rosiglitazone, on peritoneal dialysis solution (PDS)induced injuries in rat peritoneal mesothelial cells (RPMCs). RPMCs were cultured for different durations and with different concentrations of PDS. The gene expression levels of aquaporin1 (AQP1) and zonula occluden1 (ZO1) were determined using reverse transcriptionquantitative polymerase chain reaction analysis. The protein levels of AQP1, ZO1 and PPARgamma were measured using western blot analysis. Interleukin (IL)6 and IL8 were detected using ELISA. The RPMCs were damaged by stimulation with 4.25% PDS for 72 h. The expression levels of AQP1 and ZO1 were increased, and the secretion of IL6 and IL8 were decreased by Rosiglitazone. The use of the PPARgamma inhibitor, GW9662, completely prevented the effects of Rosiglitazone. These results indicated that PDS exposure stimulated an inflammatory response in the RPMCs. The PPARgamma activator, Rosiglitazone, appeared to relieve the injury by inhibiting inflammation, and regulating the expression of AQP1 and ZO1, however further investigations are required to elucidate the potential underlying mechanism.
Rosiglitazone drives cavin-2/SDPR expression in adipocytes in a CEBPalpha-dependent manner.[Pubmed:28278164]
PLoS One. 2017 Mar 9;12(3):e0173412.
Caveolae are abundant adipocyte surface domains involved in insulin signaling, membrane trafficking and lipid homeostasis. Transcriptional control mechanisms for caveolins and cavins, the building blocks of caveolae, are thus arguably important for adipocyte biology and studies in this area may give insight into insulin resistance and diabetes. Here we addressed the hypothesis that one of the less characterized caveolar components, cavin-2 (SDPR), is controlled by CCAAT/Enhancer Binding Protein (CEBPalpha) and Peroxisome Proliferator-Activated Receptor Gamma (PPARG). Using human mRNA expression data we found that SDPR correlated with PPARG in several tissues. This was also observed during differentiation of 3T3-L1 fibroblasts into adipocytes. Treatment of 3T3-L1-derived adipocytes with the PPARgamma-activator Rosiglitazone increased SDPR and CEBPalpha expression at both the mRNA and protein levels. Silencing of CEBPalpha antagonized these effects. Further, adenoviral expression of PPARgamma/CEBPalpha or Rosiglitazone-treatment increased SDPR expression in primary rat adipocytes. The myocardin family coactivator MKL1 was recently shown to regulate SDPR expression in human coronary artery smooth muscle cells. However, we found that actin depolymerization, known to inhibit MKL1 and MKL2, was without effect on SDPR mRNA levels in adipocytes, even though overexpression of MKL1 and MKL2 had the capacity to increase caveolins and cavins and to repress PPARgamma/CEBPalpha. Altogether, this work demonstrates that CEBPalpha expression and PPARgamma-activity promote SDPR transcription and further supports the emerging notion that PPARgamma/CEBPalpha and MKL1/MKL2 are antagonistic in adipocytes.
Preventive effect of rosiglitazone on liver injury in a mouse model of decompression sickness.[Pubmed:28357820]
Diving Hyperb Med. 2017 Mar;47(1):17-23.
BACKGROUND AND AIMS: Severe decompression sickness (DCS) is a multi-organ injury. This study investigated the preventive effects of Rosiglitazone on liver injury following rapid decompression in mice and examined the underlying mechanisms. METHODS: Mice were randomly divided into four groups: a control group, vehicle group, and Rosiglitazone (5 and 10 mg.kg(-)(1)) groups, the latter three being exposed to a pressure of 911 kPa. Haematoxylin and eosin staining, plasma levels of alanine transaminase (ALT), aspartate transaminase (AST) and lactate dehydrogenase and blood cell counts were used to evaluate liver injury at 30 min after rapid decompression. The expression of endothelial and inducible nitric oxide synthase (iNOS) and its phosphorylation were measured to uncover the underlying molecular mechanisms. RESULTS: A significant increase in plasma ALT, red blood cells and platelets, and a decrease in neutrophils were observed in the vehicle group. Furthermore, the expression of iNOS, E-selectin and the total level of NO in hepatic tissue, and soluble E-selectin in the plasma were significantly elevated in the vehicle group. Rosiglitazone pre-treatment prevented the increases in ALT (and AST), soluble E-selectin concentration, red blood cells and platelet counts. Moreover, Rosiglitazone reduced over-expression of iNOS and the NO level, prevented the fall in neutrophil count and promoted the phosphorylation of iNOS in the liver. CONCLUSIONS: Pre-treatment with Rosiglitazone ameliorated liver injury from severe DCS. This preventive effect may be partly mediated by stimulating endothelial NO production, improving endothelial function and limiting inflammatory processes.
Rosiglitazone Regulates TLR4 and Rescues HO-1 and NRF2 Expression in Myometrial and Decidual Macrophages in Inflammation-Induced Preterm Birth.[Pubmed:28322133]
Reprod Sci. 2017 Dec;24(12):1590-1599.
INTRODUCTION: Elevated inflammation accounts for approximately 30% of preterm birth (PTB) cases. We previously reported that targeting the peroxisome proliferator-activated receptor gamma (PPARgamma) pathway reduced the incidence of PTB in the mouse model of endotoxin-induced PTB. The PPARgamma has proven anti-inflammatory functions and its activation via Rosiglitazone significantly downregulated the systemic inflammatory response and reduced PTB and stillbirth rate by 30% and 41%, respectively, in our model. Oxidative stress is inseparable from inflammation, and Rosiglitazone has a reported antioxidative activity. In the current study, we therefore aimed to evaluate whether Rosiglitazone treatment had effects outside of inflammatory pathway, specifically on the antioxidation pathway in our model. METHODS: Pregnant C57BL/6J mice (E16.5) were treated with phosphate-buffered saline (PBS), Rosiglitazone (Rosi), lipopolysaccharide (LPS; 10microg in 200microL 1XPBS), or LPS + Rosi (6 hours after the LPS injection). The myometrial and decidual tissues were collected and processed for macrophage isolation using magnetic cell sorting and F4/80+ antibody. Expression levels of antioxidative factors- Nrf2 and Ho-1-along with the LPS receptor Tlr4 were quantified by quantitative polymerase chain reaction. The protein levels were assessed by immunofluorescence staining. RESULTS: Both the decidual and myometrial macrophages from the LPS-treated animals showed significantly lowered expression of Ho-1 and Nrf2 and higher expression of Tlr4 when compared to the PBS control group. The macrophages from the animals in the LPS + Rosi group had significantly elevated expression of Ho-1 and Nrf2 and downregulated expression of Tlr4 when compared to the LPS group. CONCLUSION: Rosiglitazone administration prevents PTB by downregulating inflammation and upregulating antioxidative response.
An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma).[Pubmed:7768881]
J Biol Chem. 1995 Jun 2;270(22):12953-6.
Thiazolidinedione derivatives are antidiabetic agents that increase the insulin sensitivity of target tissues in animal models of non-insulin-dependent diabetes mellitus. In vitro, thiazolidinediones promote adipocyte differentiation of preadipocyte and mesenchymal stem cell lines; however, the molecular basis for this adipogenic effect has remained unclear. Here, we report that thiazolidinediones are potent and selective activators of peroxisome proliferator-activated receptor gamma (PPAR gamma), a member of the nuclear receptor superfamily recently shown to function in adipogenesis. The most potent of these agents, BRL49653, binds to PPAR gamma with a Kd of approximately 40 nM. Treatment of pluripotent C3H10T1/2 stem cells with BRL49653 results in efficient differentiation to adipocytes. These data are the first demonstration of a high affinity PPAR ligand and provide strong evidence that PPAR gamma is a molecular target for the adipogenic effects of thiazolidinediones. Furthermore, these data raise the intriguing possibility that PPAR gamma is a target for the therapeutic actions of this class of compounds.


