Gentiside BCAS# 1225022-67-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1225022-67-2 | SDF | Download SDF |
| PubChem ID | 46229236 | Appearance | Powder |
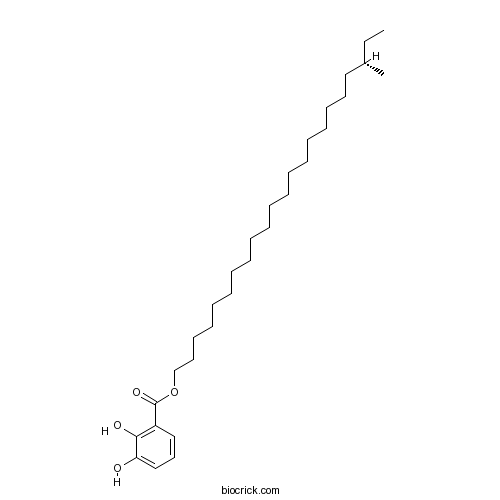
| Formula | C30H52O4 | M.Wt | 476.74 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(20S)-20-methyldocosyl] 2,3-dihydroxybenzoate | ||
| SMILES | CCC(C)CCCCCCCCCCCCCCCCCCCOC(=O)C1=C(C(=CC=C1)O)O | ||
| Standard InChIKey | FIEXPCXAWNKDMT-SANMLTNESA-N | ||
| Standard InChI | InChI=1S/C30H52O4/c1-3-26(2)22-19-17-15-13-11-9-7-5-4-6-8-10-12-14-16-18-20-25-34-30(33)27-23-21-24-28(31)29(27)32/h21,23-24,26,31-32H,3-20,22,25H2,1-2H3/t26-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Gentiside B is a potent inducer of neurite outgrowth on PC12 cells, it shows a significant neuritogenic activity at 30 microM against PC12 cells that is comparable to that seen for the best nerve growth factor (NGF) concentration of 40 ng/mL. |

Gentiside B Dilution Calculator

Gentiside B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0976 mL | 10.4879 mL | 20.9758 mL | 41.9516 mL | 52.4395 mL |
| 5 mM | 0.4195 mL | 2.0976 mL | 4.1952 mL | 8.3903 mL | 10.4879 mL |
| 10 mM | 0.2098 mL | 1.0488 mL | 2.0976 mL | 4.1952 mL | 5.2439 mL |
| 50 mM | 0.042 mL | 0.2098 mL | 0.4195 mL | 0.839 mL | 1.0488 mL |
| 100 mM | 0.021 mL | 0.1049 mL | 0.2098 mL | 0.4195 mL | 0.5244 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- INK 128 (MLN0128)
Catalog No.:BCC3880
CAS No.:1224844-38-5
- GBLD 345
Catalog No.:BCC6787
CAS No.:122479-08-7
- 4-O-(3-nitropropanoyl)corollin
Catalog No.:BCC8716
CAS No.:122475-42-7
- MFZ 10-7
Catalog No.:BCC6180
CAS No.:1224431-15-5
- Paederosidic acid methyl ester
Catalog No.:BCN3439
CAS No.:122413-01-8
- Amylin
Catalog No.:BCC6017
CAS No.:122384-88-7
- Rosiglitazone
Catalog No.:BCC2264
CAS No.:122320-73-4
- 6''-O-acetylisovitexin
Catalog No.:BCN6114
CAS No.:1223097-20-8
- Vermisporin
Catalog No.:BCN1863
CAS No.:122301-98-8
- QL-IX-55
Catalog No.:BCC1876
CAS No.:1223002-54-7
- Torin 2
Catalog No.:BCC4606
CAS No.:1223001-51-1
- Torin 1
Catalog No.:BCC3676
CAS No.:1222998-36-8
- Naltriben mesylate
Catalog No.:BCC5683
CAS No.:122517-78-6
- AG 99
Catalog No.:BCC6667
CAS No.:122520-85-8
- DCC-2618
Catalog No.:BCC1520
CAS No.:1225278-16-9
- Gelomulide A
Catalog No.:BCN6580
CAS No.:122537-59-1
- Gelomulide B
Catalog No.:BCN6588
CAS No.:122537-60-4
- SKLB1002
Catalog No.:BCC4312
CAS No.:1225451-84-2
- Esculentoside T
Catalog No.:BCC1077
CAS No.:
- Ajugamarin G1
Catalog No.:BCN3659
CAS No.:122587-83-1
- Ajugamarin F4
Catalog No.:BCN3656
CAS No.:122587-84-2
- 11-Hydroxyrankinidine
Catalog No.:BCN4814
CAS No.:122590-03-8
- 11-Hydroxyhumantenine
Catalog No.:BCN4863
CAS No.:122590-04-9
- Garbanzol
Catalog No.:BCN6811
CAS No.:1226-22-8
Gentisides C-K: nine new neuritogenic compounds from the traditional Chinese medicine Gentiana rigescens Franch.[Pubmed:20813533]
Bioorg Med Chem. 2010 Oct 1;18(19):6995-7000.
Nine new alkyl 2,3-dihydroxybenzoates, gentisides C-K, were isolated from the traditional Chinese medicine Gentiana rigescens Franch. Their structures and stereochemistry were elucidated by spectroscopic methods, and comparison of the specific rotation with that of the Gentiside B. These metabolites are additional members of the gentisides which belong to a novel class of neuritogenic compounds. They are structurally different from one another because they possess varying alkyl chain lengths, with or without an isobutyl or isopropyl group at the end of the alkyl chain. These compounds are potent inducers of neurite outgrowth on PC12 cells. The gentiside C possessing the shortest alkyl chain length exhibited the highest neuritogenic activity among all of the gentisides. Gentiside C showed a significant neuritogenic activity at 1 muM against PC12 cells comparable to that seen for the best nerve growth factor (NGF) concentration of 40 ng/mL. In addition, evident neuritogenic activity was observed in the cells when treated with gentiside C at a concentration as low as 0.03 muM. The structure-activity relationships within the gentisides A-K revealed that alkyl chain length is important for the activity, but structure diversity at the end of the alkyl chain is not.


