GarbanzolCAS# 1226-22-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1226-22-8 | SDF | File under preparation. |
| PubChem ID | 442410 | Appearance | Powder |
| Formula | C15H12O5 | M.Wt | 272.25 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
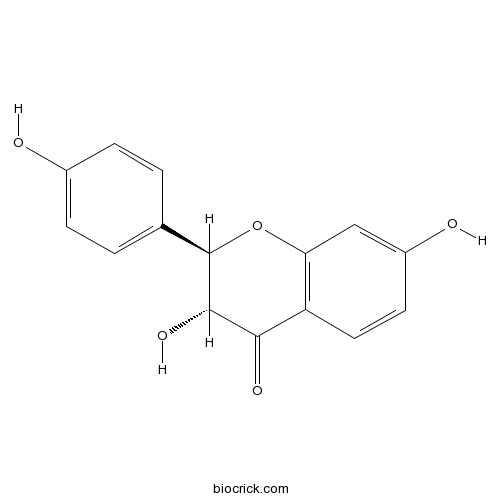
| Chemical Name | (2R,3R)-3,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one | ||
| SMILES | C1=CC(=CC=C1C2C(C(=O)C3=C(O2)C=C(C=C3)O)O)O | ||
| Standard InChIKey | VRTGGIJPIYOHGT-LSDHHAIUSA-N | ||
| Standard InChI | InChI=1S/C15H12O5/c16-9-3-1-8(2-4-9)15-14(19)13(18)11-6-5-10(17)7-12(11)20-15/h1-7,14-17,19H/t14-,15+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Garbanzol is a natural product from Cicer arietinum. |
| In vitro | Antioxidant capacity and identification of the constituents of ethyl acetate fraction from Rhus verniciflua Stokes by HPLC-MS.[Pubmed: 28100074 ]Nat Prod Res. 2017 Jul;31(13):1573-1577.Ethyl acetate fraction (EAF) from Rhus verniciflua Stokes is an important source of bioactive compounds. The aim of this study was the tentative identification and quantification of phenolic compounds, comparison of the phenolic structure-antioxidant activity relationships.
|
| Structure Identification | Metab Eng. 2015 Sep;31:84-93.Assembly of a novel biosynthetic pathway for production of the plant flavonoid fisetin in Escherichia coli.[Pubmed: 26192693 ]Plant secondary metabolites are an underutilized pool of bioactive molecules for applications in the food, pharma and nutritional industries. One such molecule is fisetin, which is present in many fruits and vegetables and has several potential health benefits, including anti-cancer, anti-viral and anti-aging activity. Moreover, fisetin has recently been shown to prevent Alzheimer's disease in mice and to prevent complications associated with diabetes type I. Thus far the biosynthetic pathway of fisetin in plants remains elusive.
Nat Prod Res. 2015;29(12):1177-9.Radical scavenging activities of flavonoids from roots of Akschindlium godefroyanum.[Pubmed: 25426867 ]
|

Garbanzol Dilution Calculator

Garbanzol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6731 mL | 18.3655 mL | 36.7309 mL | 73.4619 mL | 91.8274 mL |
| 5 mM | 0.7346 mL | 3.6731 mL | 7.3462 mL | 14.6924 mL | 18.3655 mL |
| 10 mM | 0.3673 mL | 1.8365 mL | 3.6731 mL | 7.3462 mL | 9.1827 mL |
| 50 mM | 0.0735 mL | 0.3673 mL | 0.7346 mL | 1.4692 mL | 1.8365 mL |
| 100 mM | 0.0367 mL | 0.1837 mL | 0.3673 mL | 0.7346 mL | 0.9183 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 11-Hydroxyhumantenine
Catalog No.:BCN4863
CAS No.:122590-04-9
- 11-Hydroxyrankinidine
Catalog No.:BCN4814
CAS No.:122590-03-8
- Ajugamarin F4
Catalog No.:BCN3656
CAS No.:122587-84-2
- Ajugamarin G1
Catalog No.:BCN3659
CAS No.:122587-83-1
- Esculentoside T
Catalog No.:BCC1077
CAS No.:
- SKLB1002
Catalog No.:BCC4312
CAS No.:1225451-84-2
- Gelomulide B
Catalog No.:BCN6588
CAS No.:122537-60-4
- Gelomulide A
Catalog No.:BCN6580
CAS No.:122537-59-1
- DCC-2618
Catalog No.:BCC1520
CAS No.:1225278-16-9
- AG 99
Catalog No.:BCC6667
CAS No.:122520-85-8
- Naltriben mesylate
Catalog No.:BCC5683
CAS No.:122517-78-6
- Gentiside B
Catalog No.:BCN7301
CAS No.:1225022-67-2
- p-Anisil
Catalog No.:BCC9112
CAS No.:1226-42-2
- Thiazovivin
Catalog No.:BCC2525
CAS No.:1226056-71-8
- PKC fragment (530-558)
Catalog No.:BCC5830
CAS No.:122613-29-0
- Ajugamarin H1
Catalog No.:BCN3658
CAS No.:122616-88-0
- Ibutilide Fumarate
Catalog No.:BCC5076
CAS No.:122647-32-9
- MK3102
Catalog No.:BCC6417
CAS No.:1226781-44-7
- Norpterosin B glucoside
Catalog No.:BCN7302
CAS No.:1226785-88-1
- Norpterosin B
Catalog No.:BCN7101
CAS No.:1226892-20-1
- FLLL32
Catalog No.:BCC6499
CAS No.:1226895-15-3
- ATB-346
Catalog No.:BCC5289
CAS No.:1226895-20-0
- SB 277011A dihydrochloride
Catalog No.:BCC7887
CAS No.:1226917-67-4
- BAY 87-2243
Catalog No.:BCC4131
CAS No.:1227158-85-1
Assembly of a novel biosynthetic pathway for production of the plant flavonoid fisetin in Escherichia coli.[Pubmed:26192693]
Metab Eng. 2015 Sep;31:84-93.
Plant secondary metabolites are an underutilized pool of bioactive molecules for applications in the food, pharma and nutritional industries. One such molecule is fisetin, which is present in many fruits and vegetables and has several potential health benefits, including anti-cancer, anti-viral and anti-aging activity. Moreover, fisetin has recently been shown to prevent Alzheimer's disease in mice and to prevent complications associated with diabetes type I. Thus far the biosynthetic pathway of fisetin in plants remains elusive. Here, we present the heterologous assembly of a novel fisetin pathway in Escherichia coli. We propose a novel biosynthetic pathway from the amino acid, tyrosine, utilizing nine heterologous enzymes. The pathway proceeds via the synthesis of two flavanones never produced in microorganisms before--Garbanzol and resokaempferol. We show for the first time a functional biosynthetic pathway and establish E. coli as a microbial platform strain for the production of fisetin and related flavonols.
Antioxidant capacity and identification of the constituents of ethyl acetate fraction from Rhus verniciflua Stokes by HPLC-MS.[Pubmed:28100074]
Nat Prod Res. 2017 Jul;31(13):1573-1577.
Ethyl acetate fraction (EAF) from Rhus verniciflua Stokes is an important source of bioactive compounds. The aim of this study was the tentative identification and quantification of phenolic compounds, comparison of the phenolic structure-antioxidant activity relationships. Twelve compounds of EAF belonging to polyphenol types were detected by high performance liquid chromatography and analysed on line with negative ion electrospray ionisation tandem mass spectrometry, which were ethoxy 3-hydroxy benzoic acid, gallic acid (GA), 3,4-dihydroxy amygdalic acid, gallic acid cetyl ester, protocatechuic acid (PA), fustin, ethyl gallate (EG), Garbanzol, fisetin, sulfuretin, butin and 3,7-dihydroxyflavanone-4'-rhamnoside. The antioxidant activity were evaluated based on the different types of radical scavenging capacities, i.e. DPPH., ABTS.+ and OH. The antioxidant capacity of EAF mainly depended on the GA, EG, PA, fisetin, sulfuretin and butin. The phenolics exhibited a dose-dependent behaviour and high antioxidant ability.
Radical scavenging activities of flavonoids from roots of Akschindlium godefroyanum.[Pubmed:25426867]
Nat Prod Res. 2015;29(12):1177-9.
Chemical constituents of crude ethyl acetate extract of roots of Akschindlium godefroyanum (Kuntze) H. Ohashi were investigated and seven flavonoids were isolated. Their structures were identified based on spectroscopic methods as well as by comparison with spectral data reported in the literature as six flavanonols and a flavonol including 7,4'-dihydroxy-5,3'-dimethoxyflavanonol (1), neophellamuretin (2), taxifolin (3), erycibenin D (4), geraldol (5), fustin (6) and Garbanzol (7). Compounds 2, 4 and 7 were found in the genus Akschindlium for the first time. Compounds 3, 5 and 6 appeared to have free radical scavenging activities using DPPH assay with IC50 of 21, 40 and 15 mug/mL, respectively.


