SB 277011A dihydrochlorideSelective D3 antagonist CAS# 1226917-67-4 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1226917-67-4 | SDF | Download SDF |
| PubChem ID | 75358288 | Appearance | Powder |
| Formula | C28H32Cl2N4O | M.Wt | 511.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in water and to 50 mM in DMSO | ||
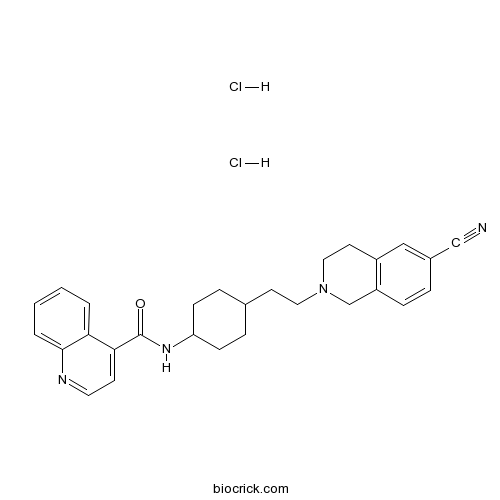
| Chemical Name | N-[4-[2-(6-cyano-3,4-dihydro-1H-isoquinolin-2-yl)ethyl]cyclohexyl]quinoline-4-carboxamide;dihydrochloride | ||
| SMILES | C1CC(CCC1CCN2CCC3=C(C2)C=CC(=C3)C#N)NC(=O)C4=CC=NC5=CC=CC=C45.Cl.Cl | ||
| Standard InChIKey | HEZIOTGUXSPDAK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H30N4O.2ClH/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27;;/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33);2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective dopamine D3 receptor antagonist (pKi values are 8.0, 6.0, 5.0 and <5.2 for D3, D2, 5-HT1D and 5-HT1B respectively). Brain penetrant. |

SB 277011A dihydrochloride Dilution Calculator

SB 277011A dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9551 mL | 9.7754 mL | 19.5507 mL | 39.1014 mL | 48.8768 mL |
| 5 mM | 0.391 mL | 1.9551 mL | 3.9101 mL | 7.8203 mL | 9.7754 mL |
| 10 mM | 0.1955 mL | 0.9775 mL | 1.9551 mL | 3.9101 mL | 4.8877 mL |
| 50 mM | 0.0391 mL | 0.1955 mL | 0.391 mL | 0.782 mL | 0.9775 mL |
| 100 mM | 0.0196 mL | 0.0978 mL | 0.1955 mL | 0.391 mL | 0.4888 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ATB-346
Catalog No.:BCC5289
CAS No.:1226895-20-0
- FLLL32
Catalog No.:BCC6499
CAS No.:1226895-15-3
- Norpterosin B
Catalog No.:BCN7101
CAS No.:1226892-20-1
- Norpterosin B glucoside
Catalog No.:BCN7302
CAS No.:1226785-88-1
- MK3102
Catalog No.:BCC6417
CAS No.:1226781-44-7
- Ibutilide Fumarate
Catalog No.:BCC5076
CAS No.:122647-32-9
- Ajugamarin H1
Catalog No.:BCN3658
CAS No.:122616-88-0
- PKC fragment (530-558)
Catalog No.:BCC5830
CAS No.:122613-29-0
- Thiazovivin
Catalog No.:BCC2525
CAS No.:1226056-71-8
- p-Anisil
Catalog No.:BCC9112
CAS No.:1226-42-2
- Garbanzol
Catalog No.:BCN6811
CAS No.:1226-22-8
- 11-Hydroxyhumantenine
Catalog No.:BCN4863
CAS No.:122590-04-9
- BAY 87-2243
Catalog No.:BCC4131
CAS No.:1227158-85-1
- 4-Fluoro-1-(3-(pyrimidin-5-yl)phenyl)-1-(2-(trifluoromethyl)pyridin-4-yl)-1H-isoindol-3-amine
Catalog No.:BCC5113
CAS No.:1227163-56-5
- AZD3839
Catalog No.:BCC6471
CAS No.:1227163-84-9
- Liangshanin A
Catalog No.:BCN6115
CAS No.:122717-54-8
- Philanthotoxin 74
Catalog No.:BCC7478
CAS No.:1227301-51-0
- Bi-linderone
Catalog No.:BCN6116
CAS No.:1227375-09-8
- Deltorphin I
Catalog No.:BCC6233
CAS No.:122752-15-2
- [D-Ala2]-Deltorphin II
Catalog No.:BCC5723
CAS No.:122752-16-3
- StemRegenin 1 (SR1)
Catalog No.:BCC3637
CAS No.:1227633-49-9
- A 943931 dihydrochloride
Catalog No.:BCC7772
CAS No.:1227675-50-4
- SCH 39166 hydrobromide
Catalog No.:BCC7317
CAS No.:1227675-51-5
- MNI-caged-NMDA
Catalog No.:BCC5888
CAS No.:1227675-52-6
Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A.[Pubmed:10945872]
J Pharmacol Exp Ther. 2000 Sep;294(3):1154-65.
SB-277011-A (trans-N-[4-[2-(6-cyano-1,2,3, 4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolininecarboxamide), is a brain-penetrant, high-affinity, and selective dopamine D(3) receptor antagonist. Radioligand-binding experiments in Chinese hamster ovary (CHO) cells transfected with human dopamine D(3) or D(2 long) (hD(3), hD(2)) receptors showed SB-277011-A to have high affinity for the hD(3) receptor (pK(i) = 7.95) with 100-fold selectivity over the hD(2) receptor and over 66 other receptors, enzymes, and ion channels. Similar radioligand-binding data for SB-277011-A were obtained from CHO cells transfected with rat dopamine D(3) or D(2). In the microphysiometer functional assay, SB-277011-A antagonized quinpirole-induced increases in acidification in CHO cells overexpressing the hD(3) receptor (pK(b) = 8.3) and was 80-fold selective over hD(2) receptors. Central nervous system penetration studies showed that SB-277011-A readily entered the brain. In in vivo microdialysis studies, SB-277011-A (2. 8 mg/kg p.o.) reversed the quinelorane-induced reduction of dopamine efflux in the nucleus accumbens but not striatum, a regional selectivity consistent with the distribution of the dopamine D(3) receptor in rat brain. SB-277011-A (2-42.3 mg/kg p.o.) did not affect spontaneous locomotion, or stimulant-induced hyperlocomotion. SB-277011-A (4.1-42.2 mg/kg p.o.) did not reverse prepulse inhibition deficits in apomorphine- or quinpirole-treated rats, but did significantly reverse the prepulse inhibition deficit in isolation-reared rats at a dose of 3 mg/kg p.o. SB-277011-A (2.5-78. 8 mg/kg p.o.) was noncataleptogenic and did not raise plasma prolactin levels. Thus, dopamine D(3) receptor blockade produces few of the behavioral effects characteristic of nonselective dopamine receptor antagonists. The effect of SB-277011-A on isolation-induced prepulse inhibition deficit suggests that blockade of dopamine D(3) receptors may benefit the treatment of schizophrenia.
Design and synthesis of trans-N-[4-[2-(6-cyano-1,2,3, 4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide (SB-277011): A potent and selective dopamine D(3) receptor antagonist with high oral bioavailability and CNS penetration in the rat.[Pubmed:10794704]
J Med Chem. 2000 May 4;43(9):1878-85.
A selective dopamine D(3) receptor antagonist offers the potential for an effective antipsychotic therapy, free of the serious side effects of currently available drugs. Using clearance and brain penetration studies as a screen, a series of 1,2,3, 4-tetrahydroisoquinolines, exemplified by 13, was identified with high D(3) affinity and selectivity against the D(2) receptor. Following examination of molecular models, the flexible butyl linker present in 13 was replaced by a more conformationally constrained cyclohexylethyl linker, leading to compounds with improved oral bioavailability and selectivity over other receptors. Subsequent optimization of this new series to improve the cytochrome P450 inhibitory profile and CNS penetration gave trans-N-[4-[2-(6-cyano-1, 2,3, 4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarbo xamide (24, SB-277011). This compound is a potent and selective dopamine D(3) receptor antagonist with high oral bioavailability and brain penetration in the rat and represents an excellent new chemical tool for the investigation of the role of the dopamine D(3) receptor in the CNS.


