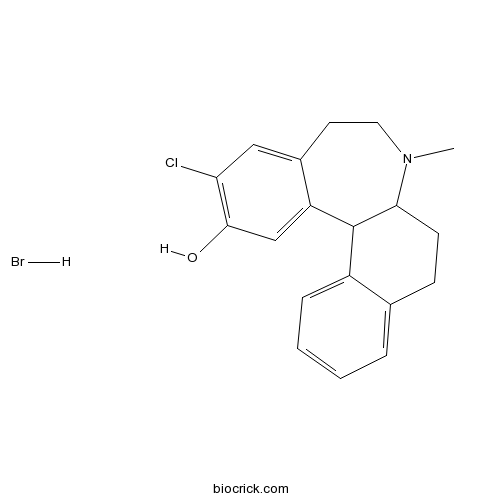
SCH 39166 hydrobromideHigh affinity D1/D5 receptor antagonist CAS# 1227675-51-5 |
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- VX-680 (MK-0457,Tozasertib)
Catalog No.:BCC2167
CAS No.:639089-54-6
- Reversine
Catalog No.:BCC1892
CAS No.:656820-32-5
- AZD1152
Catalog No.:BCC1393
CAS No.:722543-31-9
- XL228
Catalog No.:BCC2058
CAS No.:898280-07-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1227675-51-5 | SDF | Download SDF |
| PubChem ID | 73324728 | Appearance | Powder |
| Formula | C19H21BrClNO | M.Wt | 394.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ecopipam hydrobromide | ||
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | 11-chloro-7-methyl-5,6,6a,8,9,13b-hexahydronaphtho[1,2-a][3]benzazepin-12-ol;hydrobromide | ||
| SMILES | CN1CCC2=CC(=C(C=C2C3C1CCC4=CC=CC=C34)O)Cl.Br | ||
| Standard InChIKey | GAUWIDFICGEZKR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H20ClNO.BrH/c1-21-9-8-13-10-16(20)18(22)11-15(13)19-14-5-3-2-4-12(14)6-7-17(19)21;/h2-5,10-11,17,19,22H,6-9H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity dopamine D1/D5 receptor antagonist; displays Ki values of 1.2, 2, 980, 5520, 80 and 731 nM for binding to D1, D5, D2, D4, 5-HT and α2a receptors, respectively. |

SCH 39166 hydrobromide Dilution Calculator

SCH 39166 hydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5334 mL | 12.6669 mL | 25.3338 mL | 50.6675 mL | 63.3344 mL |
| 5 mM | 0.5067 mL | 2.5334 mL | 5.0668 mL | 10.1335 mL | 12.6669 mL |
| 10 mM | 0.2533 mL | 1.2667 mL | 2.5334 mL | 5.0668 mL | 6.3334 mL |
| 50 mM | 0.0507 mL | 0.2533 mL | 0.5067 mL | 1.0134 mL | 1.2667 mL |
| 100 mM | 0.0253 mL | 0.1267 mL | 0.2533 mL | 0.5067 mL | 0.6333 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- A 943931 dihydrochloride
Catalog No.:BCC7772
CAS No.:1227675-50-4
- StemRegenin 1 (SR1)
Catalog No.:BCC3637
CAS No.:1227633-49-9
- [D-Ala2]-Deltorphin II
Catalog No.:BCC5723
CAS No.:122752-16-3
- Deltorphin I
Catalog No.:BCC6233
CAS No.:122752-15-2
- Bi-linderone
Catalog No.:BCN6116
CAS No.:1227375-09-8
- Philanthotoxin 74
Catalog No.:BCC7478
CAS No.:1227301-51-0
- Liangshanin A
Catalog No.:BCN6115
CAS No.:122717-54-8
- AZD3839
Catalog No.:BCC6471
CAS No.:1227163-84-9
- 4-Fluoro-1-(3-(pyrimidin-5-yl)phenyl)-1-(2-(trifluoromethyl)pyridin-4-yl)-1H-isoindol-3-amine
Catalog No.:BCC5113
CAS No.:1227163-56-5
- BAY 87-2243
Catalog No.:BCC4131
CAS No.:1227158-85-1
- SB 277011A dihydrochloride
Catalog No.:BCC7887
CAS No.:1226917-67-4
- ATB-346
Catalog No.:BCC5289
CAS No.:1226895-20-0
- MNI-caged-NMDA
Catalog No.:BCC5888
CAS No.:1227675-52-6
- CEP-32496 hydrochloride
Catalog No.:BCC1468
CAS No.:1227678-26-3
- ACTH (1-39)
Catalog No.:BCC6028
CAS No.:12279-41-3
- GSK2334470
Catalog No.:BCC4982
CAS No.:1227911-45-6
- Gadodiamide
Catalog No.:BCC4663
CAS No.:122795-43-1
- Marinopyrrole A
Catalog No.:BCC4098
CAS No.:1227962-62-0
- Sabutoclax
Catalog No.:BCC2236
CAS No.:1228108-65-3
- 8-Geranyloxy-5,7-dimethoxycoumarin
Catalog No.:BCN6117
CAS No.:1228175-65-2
- MRS 2957 triethylammonium salt
Catalog No.:BCC6133
CAS No.:1228271-30-4
- H-D-Phe(4-F)-OH .HCl
Catalog No.:BCC3217
CAS No.:122839-52-5
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Cefoselis Sulfate
Catalog No.:BCC4769
CAS No.:122841-12-7
Dopamine D1/D5 receptor antagonists with improved pharmacokinetics: design, synthesis, and biological evaluation of phenol bioisosteric analogues of benzazepine D1/D5 antagonists.[Pubmed:15689153]
J Med Chem. 2005 Feb 10;48(3):680-93.
Benzazepines 1 and 2 (SCH 23390 and SCH 39166, respectively) are two classical benzazepine D1/D5 antagonists, with Ki values 1.4 and 1.2 nM, respectively. Compound 2 has been in human clinical trials for a variety of diseases, including schizophrenia, cocaine addition, and obesity. Both 1 and 2 displayed low plasma levels and poor oral bioavailability, due to rapid first-pass metabolism of the phenol moieties. Several heterocyclic systems containing an N-H hydrogen bond donor were synthesized and evaluated as phenol isosteres. The preference orientation of the hydrogen bond was established by comparison of analogues containing different NH vectors. Replacement of the phenol group of 2 with an indole ring generated the first potent D1/D5 antagonist 11b. Further optimization led to the synthesis of very potent benzimidazolones 19, 20 and benzothiazolone analogues 28, 29. These compounds have excellent selectivity over D2-D4 receptors, alpha2a receptor, and the 5-HT transporter. Compared to 2, these heterocyclic phenol isosteres showed much better pharmacokinetic profiles as demonstrated by rat plasma levels. In sharp contrast, similar phenolic replacements in 1 decreased the binding affinity dramatically, presumably due to a conformational change of the pendant phenyl group. However, one indazole compound 33 was identified as a potent D1/D5 ligand in this series.
A comparison of the effects of the D1 receptor antagonists SCH 23390 and SCH 39166 on suppression of feeding behavior by the D1 agonist SKF38393.[Pubmed:7862841]
Psychopharmacology (Berl). 1994 Jan;113(3-4):328-33.
The hypophagic effect of the D1 receptor agonist SKF 38393 is not dose-dependently antagonized by the D1 antagonist SCH 23390. Moreover, the receptor specificity of this interaction remains in question, since SCH 23390 has significant activity at both 5-HT2 and 5-HT1C receptors, and SKF 38393 also interacts with 5-HT1C receptors. To determine the relative significance of these actions, a comparison was made between the anorectic effects in rats of SCH 23390 (0.1-1.0 mg/kg) and the benzonaphthazepine SCH 39166 (0.1-3.0 mg/kg), a D1 antagonist with negligible affinity for 5-HT sites. Both compounds inhibited food-intake dose-dependently, with SCH 23390 being approximately twice as potent as SCH 39166. Behaviorally inactive and active doses of both antagonists were tested in combination with the D1 agonist SKF 38393 (10-56 mg/kg). Neither antagonist was able to produce more than a marginal attenuation of the agonist-induced hypophagia. This demonstrates that previous failures to reverse the behavioral actions of SKF 38393 by SCH 23390 were not due to specific actions of this particular antagonist. Finally, like SCH 23390, SCH 39166 (0.3 mg/kg) was able to attenuate fully the anorectic effects of the D1 agonist SKF 82958 (1.0 and 3.0 mg/kg), demonstrating that neither compound is intrinsically unable to block D1 receptor-mediated hypophagia. The results demonstrate the generality of the D1 antagonist-mediated effect on feeding and call into question the use of SKF 38393 as a D1 agonist in studies of feeding, and perhaps in other contexts as well.
In vivo binding of SCH 39166: a D-1 selective antagonist.[Pubmed:1826927]
J Pharmacol Exp Ther. 1991 Apr;257(1):42-9.
SCH 39166 [(-)-trans-6,7,7a,8,9,13b-hexahydro-3-chloro-2-hydroxy-N-methyl- 5H-benzo[d]naphtho-[2,1b]-azepine] has been identified previously as a potent and selective D-1 antagonist. These studies demonstrated that SCH 39166 binds to the D-1 receptor in vitro and inhibits the rat conditioned avoidance response, a test predictive of antipsychotic activity. The current study demonstrates that SCH 39166 inhibits the in vivo binding of [125I]SCH 38840 to D-1 receptors in rat striatal tissue with an ED50 of 11.67 nmol/animal or 0.016 mg/kg s.c. SCH 39166 did not inhibit the in vivo binding of [125I]SCH 38840 to rat frontal cortex, suggesting that, unlike other D-1 antagonists, SCH 39166 was not binding to 5-hydroxytryptamine (5-HT)2 receptors in vivo. The in vivo binding of SCH 39166 to D-2 receptors was studied using [3H]raclopride and demonstrated that SCH 39166 did not bind to D-2 receptors up to doses of 100 mumol/animal or approximately 150 mg/kg s.c. Further studies to determine the in vivo selectivity of SCH 39166 utilized N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) to inactivate selected neurotransmitter receptors. Preadministration of SCH 39166, at doses as low as 0.01 mg/kg s.c., produced a statistically significant protection of D-1 receptors from EEDQ inactivation. SCH 39166 produced a similar protection of 5-HT2 receptors only at the highest dose tested, 10 mg/kg s.c., whereas there was no protection of D-2 sites even at this high dose.(ABSTRACT TRUNCATED AT 250 WORDS)


