Marinopyrrole ASelective Mcl-1 inhibitor CAS# 1227962-62-0 |
- WEHI-539
Catalog No.:BCC2055
CAS No.:1431866-33-9
- Gambogic acid
Catalog No.:BCN2318
CAS No.:2752-65-0
- Obatoclax mesylate (GX15-070)
Catalog No.:BCC2234
CAS No.:803712-79-0
- ABT-263 (Navitoclax)
Catalog No.:BCC1272
CAS No.:923564-51-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1227962-62-0 | SDF | Download SDF |
| PubChem ID | 24797083 | Appearance | Powder |
| Formula | C22H12Cl4N2O4 | M.Wt | 510.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Maritoclax; (±)-Marinopyrrole | ||
| Solubility | DMSO : ≥ 43 mg/mL (84.29 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
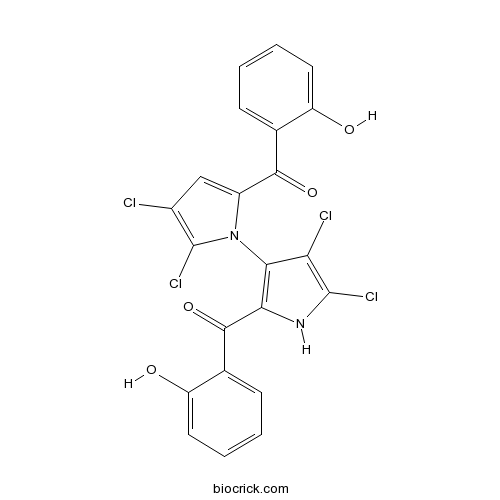
| Chemical Name | [4,5-dichloro-1-[4,5-dichloro-2-(2-hydroxybenzoyl)-1H-pyrrol-3-yl]pyrrol-2-yl]-(2-hydroxyphenyl)methanone | ||
| SMILES | C1=CC=C(C(=C1)C(=O)C2=CC(=C(N2C3=C(NC(=C3Cl)Cl)C(=O)C4=CC=CC=C4O)Cl)Cl)O | ||
| Standard InChIKey | QYPJBTMRYKRTFG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H12Cl4N2O4/c23-12-9-13(19(31)10-5-1-3-7-14(10)29)28(22(12)26)18-16(24)21(25)27-17(18)20(32)11-6-2-4-8-15(11)30/h1-9,27,29-30H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mcl-1 inhibitor; disrupts Mcl-1-Bim interaction and induces Mcl-1 proteasomal degradation. Exhibits no effect on Bcl-XL-Bim interaction. Selectively induces apoptosis in Mcl-1-dependent K562 leukemia cells. Sensitizes K562 and Raji cells to ABT-737-induced apoptosis. |

Marinopyrrole A Dilution Calculator

Marinopyrrole A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9602 mL | 9.801 mL | 19.6021 mL | 39.2042 mL | 49.0052 mL |
| 5 mM | 0.392 mL | 1.9602 mL | 3.9204 mL | 7.8408 mL | 9.801 mL |
| 10 mM | 0.196 mL | 0.9801 mL | 1.9602 mL | 3.9204 mL | 4.9005 mL |
| 50 mM | 0.0392 mL | 0.196 mL | 0.392 mL | 0.7841 mL | 0.9801 mL |
| 100 mM | 0.0196 mL | 0.098 mL | 0.196 mL | 0.392 mL | 0.4901 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Marinopyrrole A is a selective inhibitor of Mcl-1 with IC50 value of 10.1μM [1].
Marinopyrrole A is a natural product froma species of marine-derived streptomycetes and is reported to be an antagonist of Mcl-1. Mcl-1 is a member of the anti-apoptotic Bcl-2 family, which is a well-validated drug target for cancer treatment. NMR titration experiments show that Marinopyrrole A can directly interact with Mcl-1. It can prevent Bim-BH3 peptides from binding to Mcl-1 but not Bcl-XL. The cell based assay shows a high selectivity of Marinopyrrole A. Treatment with Marinopyrrole A inhibit the viability of K562 cells transfected with Mcl-1 gene with EC50 value of 1.6μM. The selectivity is more than 40-fold greater over the cells transfected with Bcl-XL gene. Moreover, Marinopyrrole A can decreases Mcl-1 expression by increasing the cleavage of caspase-3 and PARP. Marinopyrrole A is also reported to completely restore the sensitivity of multidrug resistant leukemia cells to ABT-737 [1].
References:
[1] Doi K, Li R, Sung SS, et al. Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. J Biol Chem. 2012 Mar, 287(13): 10224-35.
- Gadodiamide
Catalog No.:BCC4663
CAS No.:122795-43-1
- GSK2334470
Catalog No.:BCC4982
CAS No.:1227911-45-6
- ACTH (1-39)
Catalog No.:BCC6028
CAS No.:12279-41-3
- CEP-32496 hydrochloride
Catalog No.:BCC1468
CAS No.:1227678-26-3
- MNI-caged-NMDA
Catalog No.:BCC5888
CAS No.:1227675-52-6
- SCH 39166 hydrobromide
Catalog No.:BCC7317
CAS No.:1227675-51-5
- A 943931 dihydrochloride
Catalog No.:BCC7772
CAS No.:1227675-50-4
- StemRegenin 1 (SR1)
Catalog No.:BCC3637
CAS No.:1227633-49-9
- [D-Ala2]-Deltorphin II
Catalog No.:BCC5723
CAS No.:122752-16-3
- Deltorphin I
Catalog No.:BCC6233
CAS No.:122752-15-2
- Bi-linderone
Catalog No.:BCN6116
CAS No.:1227375-09-8
- Philanthotoxin 74
Catalog No.:BCC7478
CAS No.:1227301-51-0
- Sabutoclax
Catalog No.:BCC2236
CAS No.:1228108-65-3
- 8-Geranyloxy-5,7-dimethoxycoumarin
Catalog No.:BCN6117
CAS No.:1228175-65-2
- MRS 2957 triethylammonium salt
Catalog No.:BCC6133
CAS No.:1228271-30-4
- H-D-Phe(4-F)-OH .HCl
Catalog No.:BCC3217
CAS No.:122839-52-5
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Cefoselis Sulfate
Catalog No.:BCC4769
CAS No.:122841-12-7
- Alosetron
Catalog No.:BCC1342
CAS No.:122852-42-0
- Alosetron (Z)-2-butenedioate
Catalog No.:BCC1343
CAS No.:122852-43-1
- Alosetron Hydrochloride
Catalog No.:BCC1344
CAS No.:122852-69-1
- Panaxyne
Catalog No.:BCN6462
CAS No.:122855-49-6
- GS-9620
Catalog No.:BCC1602
CAS No.:1228585-88-3
- TAK-632
Catalog No.:BCC3639
CAS No.:1228591-30-7
Design, synthesis and evaluation of marinopyrrole derivatives as selective inhibitors of Mcl-1 binding to pro-apoptotic Bim and dual Mcl-1/Bcl-xL inhibitors.[Pubmed:25437618]
Eur J Med Chem. 2015 Jan 27;90:315-331.
Inhibition of anti-apoptotic Mcl-1 is a promising anticancer strategy to overcome the survival and chemoresistance of a broad spectrum of human cancers. We previously reported on the identification of a natural product Marinopyrrole A (1) that induces apoptosis in Mcl-1-dependent cells through Mcl-1 degradation. Here, we report the design and synthesis of novel marinopyrrole-based analogs and their evaluation as selective inhibitors of Mcl-1 as well as dual Mcl-1/Bcl-xL inhibitors. The most selective Mcl-1 antagonists were 34, 36 and 37 with 16-, 13- and 9-fold more selectivity for disrupting Mcl-1/Bim over Bcl-xL/Bim binding, respectively. Among the most potent dual inhibitors is 42 which inhibited Mcl-1/Bim and Bcl-xL/Bim binding 15-fold (IC50 = 600 nM) and 33-fold (500 nM) more potently than (+/-)-Marinopyrrole A (1), respectively. Fluorescence quenching, NMR analysis and molecular docking indicated binding of marinopyrroles to the BH3 binding site of Mcl-1. Several marinopyrroles potently decreased Mcl-1 cellular levels and induced caspase 3 activation in human breast cancer cells. Our studies provide novel "lead" marinopyrroles for further optimization as selective Mcl-1 inhibitors and dual Mcl-1 and Bcl-xL inhibitors.
De-orphaning the marine natural product (+/-)-marinopyrrole A by computational target prediction and biochemical validation.[Pubmed:28154844]
Chem Commun (Camb). 2017 Feb 14;53(14):2272-2274.
Exploring the full potential of bioactive natural products and phenotypic screening hits for drug discovery and design requires profound understanding of the macromolecular targets involved. We present a computational method for target prediction, and showcase its practical applicability, taking the marine anticancer compound (+/-)-Marinopyrrole A as an example. With an overall accuracy of 67%, the ligand-based method employed identified the natural product as a potent glucocorticoid, cholecystokinin, and orexin receptor antagonist. The results of this study demonstrate the utility of fast computational target assessment for medicinal chemistry and chemical biology.
Marinopyrrole derivatives with sulfide spacers as selective disruptors of Mcl-1 binding to pro-apoptotic protein Bim.[Pubmed:25076060]
Mar Drugs. 2014 Jul 29;12(8):4311-25.
A series of novel marinopyrroles with sulfide and sulphone spacers were designed and synthesized. Their activity to disrupt the binding of the pro-apoptotic protein, Bim, to the pro-survival proteins, Mcl-1 and Bcl-xL, was evaluated using ELISA assays. Fluorescence-quenching (FQ) assays confirmed the direct binding of marinopyrroles to Mcl-1. Benzyl- and benzyl methoxy-containing sulfide derivatives 4 and 5 were highly potent dual Mcl-1/Bim and Bcl-xL/Bim disruptors (IC50 values of 600 and 700 nM), whereas carboxylate-containing sulfide derivative 9 exhibited 16.4-fold more selectivity for disrupting Mcl-1/Bim over Bcl-xL/Bim binding. In addition, a nonsymmetrical marinopyrrole 12 is as equally potent as the parent Marinopyrrole A (1) for disrupting both Mcl-1/Bim and Bcl-xL/Bim binding. Some of the derivatives were also active in intact human breast cancer cells where they reduced the levels of Mcl-1, induced programd cell death (apoptosis) and inhibited cell proliferation.
Proteasomal degradation of Mcl-1 by maritoclax induces apoptosis and enhances the efficacy of ABT-737 in melanoma cells.[Pubmed:24223823]
PLoS One. 2013 Nov 4;8(11):e78570.
BACKGROUND AND PURPOSE: Metastatic melanoma remains one of the most invasive and highly drug resistant cancers. The over expression of anti-apoptotic protein Mcl-1 has been associated with inferior survival, poor prognosis and chemoresistance of malignant melanoma. A BH3 mimetic, ABT-737, has demonstrated efficacy in several forms of cancers. However, the efficacy of ABT-737 depends on Mcl-1. Because the over expression of Mcl-1 is frequently observed in melanoma, specifically targeting of Mcl-1 may overcome the resistance of ABT-737. In this study, we investigated the effects of Maritoclax, a novel Mcl-1-selective inhibitor, alone and in combination with ABT-737, on the survival of human melanoma cells. EXPERIMENTAL APPROACH: For cell viability assessment we performed MTT assay. Apoptosis was determined using western blot and flow cytometric analysis. KEY RESULTS: The treatment of Maritoclax reduced the cell viability of melanoma cells with an IC50 of between 2.2-5.0 microM. Further, treatment of melanoma cells with Maritoclax showed significant decrease in Mcl-1 expression. We found that Maritoclax was able to induce apoptosis in melanoma cells in a caspase-dependent manner. Moreover, Maritoclax induced Mcl-1 degradation via the proteasome system, which was associated with its pro-apoptotic activity. We also found that Maritoclax treatment increased mitochondrial translocation of Bim and Bmf. Importantly, Maritoclax markedly enhanced the efficacy of ABT-737 against melanoma cells in both two- and three-dimensional spheroids. CONCLUSIONS AND IMPLICATIONS: Taken together, these results suggest that targeting of Mcl-1 by Maritoclax may represent a new therapeutic strategy for melanoma treatment that warrants further investigation as a single therapy or in combination with other agents such as Bcl-2 inhibitors.
Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation.[Pubmed:22311987]
J Biol Chem. 2012 Mar 23;287(13):10224-35.
The anti-apoptotic Bcl-2 family of proteins, including Bcl-2, Bcl-X(L) and Mcl-1, are well-validated drug targets for cancer treatment. Several small molecules have been designed to interfere with Bcl-2 and its fellow pro-survival family members. While ABT-737 and its orally active analog ABT-263 are the most potent and specific inhibitors to date that bind Bcl-2 and Bcl-X(L) with high affinity but have a much lower affinity for Mcl-1, they are not very effective as single agents in certain cancer types because of elevated levels of Mcl-1. Accordingly, compounds that specifically target Mcl-1 may overcome this resistance. In this study, we identified and characterized the natural product Marinopyrrole A as a novel Mcl-1-specific inhibitor and named it maritoclax. We found that maritoclax binds to Mcl-1, but not Bcl-X(L), and is able to disrupt the interaction between Bim and Mcl-1. Moreover, maritoclax induces Mcl-1 degradation via the proteasome system, which is associated with the pro-apoptotic activity of maritoclax. Importantly, maritoclax selectively kills Mcl-1-dependent, but not Bcl-2- or Bcl-X(L)-dependent, leukemia cells and markedly enhances the efficacy of ABT-737 against hematologic malignancies, including K562, Raji, and multidrug-resistant HL60/VCR, by approximately 60- to 2000-fold at 1-2 muM. Taken together, these results suggest that maritoclax represents a new class of Mcl-1 inhibitors, which antagonizes Mcl-1 and overcomes ABT-737 resistance by targeting Mcl-1 for degradation.


