AlosetronCAS# 122852-42-0 |
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Pectolinarigenin
Catalog No.:BCN5813
CAS No.:520-12-7
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- Hypaconine
Catalog No.:BCN8640
CAS No.:63238-68-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 122852-42-0 | SDF | Download SDF |
| PubChem ID | 2099 | Appearance | Powder |
| Formula | C17H18N4O | M.Wt | 294.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO | ||
| Chemical Name | 5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4-dihydropyrido[4,3-b]indol-1-one | ||
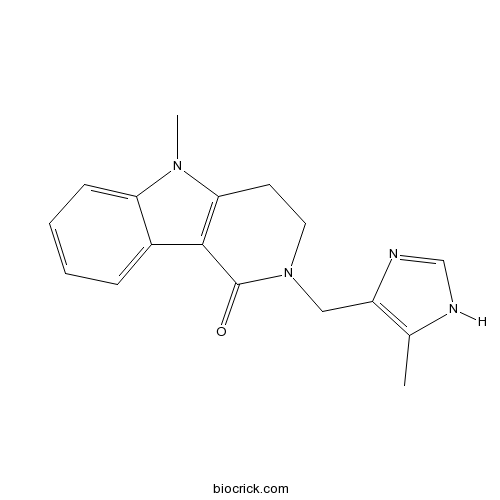
| SMILES | CC1=C(N=CN1)CN2CCC3=C(C2=O)C4=CC=CC=C4N3C | ||
| Standard InChIKey | JSWZEAMFRNKZNL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H18N4O/c1-11-13(19-10-18-11)9-21-8-7-15-16(17(21)22)12-5-3-4-6-14(12)20(15)2/h3-6,10H,7-9H2,1-2H3,(H,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Alosetron Dilution Calculator

Alosetron Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3973 mL | 16.9866 mL | 33.9732 mL | 67.9463 mL | 84.9329 mL |
| 5 mM | 0.6795 mL | 3.3973 mL | 6.7946 mL | 13.5893 mL | 16.9866 mL |
| 10 mM | 0.3397 mL | 1.6987 mL | 3.3973 mL | 6.7946 mL | 8.4933 mL |
| 50 mM | 0.0679 mL | 0.3397 mL | 0.6795 mL | 1.3589 mL | 1.6987 mL |
| 100 mM | 0.034 mL | 0.1699 mL | 0.3397 mL | 0.6795 mL | 0.8493 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Alosetron is s Serotonin 5HT3-receptor antagonist that is used in treatment of irritable bowel syndrome.
- Cefoselis Sulfate
Catalog No.:BCC4769
CAS No.:122841-12-7
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- H-D-Phe(4-F)-OH .HCl
Catalog No.:BCC3217
CAS No.:122839-52-5
- MRS 2957 triethylammonium salt
Catalog No.:BCC6133
CAS No.:1228271-30-4
- 8-Geranyloxy-5,7-dimethoxycoumarin
Catalog No.:BCN6117
CAS No.:1228175-65-2
- Sabutoclax
Catalog No.:BCC2236
CAS No.:1228108-65-3
- Marinopyrrole A
Catalog No.:BCC4098
CAS No.:1227962-62-0
- Gadodiamide
Catalog No.:BCC4663
CAS No.:122795-43-1
- GSK2334470
Catalog No.:BCC4982
CAS No.:1227911-45-6
- ACTH (1-39)
Catalog No.:BCC6028
CAS No.:12279-41-3
- CEP-32496 hydrochloride
Catalog No.:BCC1468
CAS No.:1227678-26-3
- MNI-caged-NMDA
Catalog No.:BCC5888
CAS No.:1227675-52-6
- Alosetron (Z)-2-butenedioate
Catalog No.:BCC1343
CAS No.:122852-43-1
- Alosetron Hydrochloride
Catalog No.:BCC1344
CAS No.:122852-69-1
- Panaxyne
Catalog No.:BCN6462
CAS No.:122855-49-6
- GS-9620
Catalog No.:BCC1602
CAS No.:1228585-88-3
- TAK-632
Catalog No.:BCC3639
CAS No.:1228591-30-7
- AM966
Catalog No.:BCC1355
CAS No.:1228690-19-4
- AM-095 free base
Catalog No.:BCC1352
CAS No.:1228690-36-5
- 2-Desoxy-4-epi-pulchellin
Catalog No.:BCN6118
CAS No.:122872-03-1
- Mps1-IN-2
Catalog No.:BCC4153
CAS No.:1228817-38-6
- Ziprasidone HCl
Catalog No.:BCC2511
CAS No.:122883-93-6
- MLN0905
Catalog No.:BCC3961
CAS No.:1228960-69-7
- 12-Demethylneocaesalpin F
Catalog No.:BCN6410
CAS No.:1228964-10-0
A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program.[Pubmed:24003335]
Therap Adv Gastroenterol. 2013 Sep;6(5):344-57.
OBJECTIVES: Adverse events (AEs) of ischemic colitis (IC) and complications of constipation (CoC) associated with Alosetron are rare and have been adjudicated during the first 5.5 years of the risk management program (RMP); however, changes in incidence rates relative to reductions in AE reports and increases in Alosetron prescriptions over the 9-year RMP have not been evaluated. The authors aim to evaluate temporal trends in Alosetron postmarketing safety over the 9-year RMP. METHODS: The Alosetron safety database was searched to identify cases of IC, CoC, and related AEs from 20 November 2002 to 31 December 2011. Adjudication of IC and CoC cases were based on US Food and Drug Administration-defined criteria. Incidence rates were calculated using the number of AEs and Alosetron prescriptions (expressed as cases/1000 patient-years exposure). RESULTS: A total of 29 cases were adjudicated as probable/possible IC and 7 cases were adjudicated as CoC. Cumulative adjudicated incidence rate of IC (1.03 cases/1000 patient-years) is low and stable, while that of CoC (0.25 cases/1000 patient-years) is low, declining progressively over time. Decreases in the incidence rates of potential symptoms of IC (abdominal pain with bloody diarrhea/hematochezia) and CoC (constipation) were also observed. CONCLUSIONS: Over the 9-year RMP period, incidence rates of IC and CoC remain rare. Substantial reductions over time were observed in the incidence of CoC and in symptoms suggestive of IC or CoC, while IC incidence has been stable at approximately 1.0 case/1000 patient-years. Decreases in AEs and serious outcomes associated with IC and CoC since the reintroduction of Alosetron are likely attributable to the RMP.
Application of a UPLC-MS/MS method for the analysis of alosetron in human plasma to support a bioequivalence study in healthy males and females.[Pubmed:25761551]
Biomed Chromatogr. 2015 Oct;29(10):1527-34.
A simple, rapid and sensitive ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method has been developed and validated for the determination of Alosetron (ALO) in human plasma. The assay method involved solid-phase extraction of ALO and ALO 13C-d3 as internal standard (IS) on a LichroSep DVB-HL (30 mg, 1 cm(3) ) cartridge. The chromatography was performed on an Acquity UPLC BEH C18 (50 x 2.1 mm, 1.7 microm) column using acetonitrile and 2.0 mm ammonium formate, pH 3.0 adjusted with 0.1% formic acid (80:20, v/v) as the mobile phase in an isocratic mode. For quantitative analysis, the multiple reaction monitoring transitions studied were m/z 295.1/201.0 for ALO and m/z 299.1/205.1 for IS in the positive ionization mode. The method was validated over a concentration range of 0.01-10.0 ng/mL for ALO. Post-column infusion experiment showed no positive or negative peaks in the elution range of the analyte and IS after injection of extracted blank plasma. The extent of ion-suppression/enhancement, expressed as IS-normalized matrix factor, varied from 0.96 to 1.04. The assay recovery was within 97-103% for ALO and IS. The method was successfully applied to support a bioequivalence study of 1.0 mg Alosetron tablets in 28 healthy Indian male and female subjects.
Development of a Forced Degradation Profile of Alosetron by Single Mode Reversed-Phase HPLC, LC-MS, and its Validation.[Pubmed:26839817]
Sci Pharm. 2014 Dec 29;83(2):311-20.
Determination of Alosetron in the presence of its degradation products was studied and validated by a novel HPLC method. The separation of the drug and its degradation products was achieved with the Jones Chromatography C18 analytical column (150 mm x 4.6 mm; 3 microm) with a stationary phase in isocratic elution mode. The mobile phase used was 0.01 M ammonium acetate, pH-adjusted to 3.5 with glacial acetic acid and acetonitrile in the ratio of 75:25 (V/V) at a flow rate of 1 ml/min and UV detection was carried out at 217 nm. Further, the drug was subjected to stress studies for acidic, basic, neutral, oxidative, and thermal degradations as per ICH guidelines and the drug was found to be labile in base hydrolysis and oxidation, while stable in acid, neutral, thermal, and photolytic degradation conditions. An MS study has been performed on the major degradation products to predict the degradation pathway of Alosetron. The method provided linear responses over the concentration range of 100-1500 ng/ml and regression analysis showed a correlation coefficient value (r(2)) of 0.994. The LOD and LOQ were found to be 1 ng/ml and 3 ng/ml, respectively. The developed LC method was validated as per ICH guidelines with respect to accuracy, selectivity, precision, linearity, and robustness.
Rifaximin and eluxadoline - newly approved treatments for diarrhea-predominant irritable bowel syndrome: what is their role in clinical practice alongside alosetron?[Pubmed:26559529]
Expert Opin Pharmacother. 2016;17(3):311-22.
INTRODUCTION: Diarrhea-predominant irritable bowel syndrome (IBS-D) is a common functional gastrointestinal condition in which patients experience abdominal pain, diarrhea, bloating, cramps, flatulence, fecal urgency, and incontinence. AREAS COVERED: We review two recently approved therapies that focus on treating underlying pathogenic mechanisms of IBS-D: (1) the non-absorbable antibiotic rifaximin, and (2) the opioid receptor agonist/antagonist eluxadoline. We compare the safety and efficacy data emerging from rifaximin and eluxadoline registration trials with safety and efficacy data from the Alosetron clinical development program. EXPERT OPINION: The rifaximin and eluxadoline clinical development programs for IBS-D have demonstrated significant improvement in IBS-D endpoints compared to placebo. Direct comparison of primary endpoint results from the Alosetron, rifaximin, and eluxadoline pivotal trials is not possible; however, general estimates of efficacy can be made, and these demonstrate similar and significantly greater responses to 'adequate relief' and a composite endpoint of abdominal pain/stool form for each agent compared to placebo. With the recent approval in the United States of rifaximin and eluxadoline for IBS-D, how should clinicians employ these agents? We suggest that they be utilized sequentially, taking into consideration patient symptoms and severity, prior medical history, mode of action, cost, availability, managed care coverage, and adverse event profiles.


