ABT-263 (Navitoclax)Potent Bcl-2 family inhibitor, inhibits Bcl-2, Bcl-xL, and Bcl-w CAS# 923564-51-6 |
- ABT-199
Catalog No.:BCC3614
CAS No.:1257044-40-8
- BM-1074
Catalog No.:BCC2235
CAS No.:1391108-10-3
- HA14-1
Catalog No.:BCC3593
CAS No.:65673-63-4
- Obatoclax mesylate (GX15-070)
Catalog No.:BCC2234
CAS No.:803712-79-0
- ABT-737
Catalog No.:BCC3613
CAS No.:852808-04-9
- TW-37
Catalog No.:BCC2257
CAS No.:877877-35-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 923564-51-6 | SDF | Download SDF |
| PubChem ID | 24978538 | Appearance | Powder |
| Formula | C47H55ClF3N5O6S3 | M.Wt | 974.61 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ABT-263 | ||
| Solubility | DMF : ≥ 100 mg/mL (102.61 mM) DMSO : 75 mg/mL (76.95 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
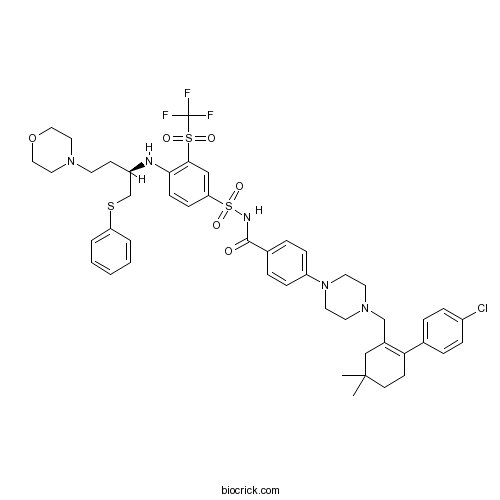
| Chemical Name | 4-[4-[[2-(4-chlorophenyl)-5,5-dimethylcyclohexen-1-yl]methyl]piperazin-1-yl]-N-[4-[[(2R)-4-morpholin-4-yl-1-phenylsulfanylbutan-2-yl]amino]-3-(trifluoromethylsulfonyl)phenyl]sulfonylbenzamide | ||
| SMILES | CC1(CCC(=C(C1)CN2CCN(CC2)C3=CC=C(C=C3)C(=O)NS(=O)(=O)C4=CC(=C(C=C4)NC(CCN5CCOCC5)CSC6=CC=CC=C6)S(=O)(=O)C(F)(F)F)C7=CC=C(C=C7)Cl)C | ||
| Standard InChIKey | JLYAXFNOILIKPP-KXQOOQHDSA-N | ||
| Standard InChI | InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | ABT-263 (Navitoclax) is a potent inhibitor of Bcl-xL, Bcl-2 and Bcl-w with Ki of ≤ 0.5 nM, ≤1 nM and ≤1 nM. | |||||
| Targets | Bcl-xL | Bcl-2 | Bcl-w | |||
| IC50 | ≤ 0.5 nM (Ki) | ≤1 nM (Ki) | ≤ 1 nM (Ki) | |||
| Cell experiment:[1] | |
| Cell lines | Murine DO11.10 T-hybridoma cells expressing murine Bcl-2, Bcl-xL and Bcl-w proteins |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | None specifc suggestion |
| Applications | ABT-263 is an antitumor effector in preclinical and early clinical studies. It binds to Bcl-2, Bcl-xL, and Bcl-w in vitro, but only targets Bcl-2 in vivo. In human non-Hodgkin lymphomas, high expression of Bcl-2 sensitized to ABT-263 elevated proapoptotic Bim. |
| Animal experiment:[2] | |
| Animal models | Immune-deficient NOD/SCID or NOD/SCID, ILγ receptor negative mice |
| Dosage form | Orally taken at 100 mg/kg/day for 21 days |
| Application | ABT-263 can largely inhibited the activity of patient-derived pediatric acute lymphoblastic leukemia xenograft. ABT-263 sensitivity was correlated with low MCL1 mRNA expression levels. BH3 profiling revealed that resistance to ABT-263 correlated with mitochondrial priming by NOXA peptide. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: 1. Mérino D1, Khaw SL, Glaser SP et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells.Blood. 2012 Jun 14;119(24):5807-16. 2. Suryani S, Carol H, Chonghaile TN et al. Cell and Molecular Determinants of In Vivo Efficacy of the BH3 Mimetic ABT-263 against Pediatric Acute Lymphoblastic Leukemia Xenografts. Clin Cancer Res. 2014 Jul 10. | |

ABT-263 (Navitoclax) Dilution Calculator

ABT-263 (Navitoclax) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0261 mL | 5.1303 mL | 10.2605 mL | 20.521 mL | 25.6513 mL |
| 5 mM | 0.2052 mL | 1.0261 mL | 2.0521 mL | 4.1042 mL | 5.1303 mL |
| 10 mM | 0.1026 mL | 0.513 mL | 1.0261 mL | 2.0521 mL | 2.5651 mL |
| 50 mM | 0.0205 mL | 0.1026 mL | 0.2052 mL | 0.4104 mL | 0.513 mL |
| 100 mM | 0.0103 mL | 0.0513 mL | 0.1026 mL | 0.2052 mL | 0.2565 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
ABT-263 tablets (100mg) orally administered to both intact and TDC dogs exhibited low clearance (0.673 ml/min per kilogram) and low volume of distribution (0.5-0.7 l/kg) with a half-life of 22.2 hours and the bioavailablity of 56.5%, where 13.5% of the total ABT-263 dose in TDC dogs was transported in lymph with the bioavailability of 21.7%. However, in fasted TDC dogs, a 1.8-fold decrease in lymph transport of ABT-263 was observed.
Abstract
As an inhibitor of Bcl-2, Bcl-x(L) and Bcl-w, ABT-263 exhibits antitumor activity against haematological tumors when used alone or in combination with other agents.
Abstract
ABT-263 is a potent and selective inhibitor of Bcl-2 and Bcl-x(L).
Abstract
Synergistic effects against ovarian cancer cells were observed in both ABT-263/paclitaxel and ABT-263/gemcitabine treatments with a stronger effect in ABT-163/paclitaxel treatment killing 50% of tested ovarian cancer cells. The combination of ABT-263 with taxane-based therapy is more suitable for ovarian cancer patients with high levels of Bcl-x(L).
Abstract
ABT-263, an inhibitor of Bcl-2, Bc,-x(L) and Bcl-w with promising antitumor efficacy, is sensitive to human non-Hodgkin lymphomas with high expression of Bcl-2.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ABT-263 is an orally sellecitive inhibitor of B-cell leukemia 2 (Bcl-2) family of proteins with potential antineoplastic activity. ABT-263 is a small molecular with the formula of C47H55ClF3N5O6S3 and Molecular Weight of 974. As a Bad-like Bh3 minetic, ABT-263 binds to Bcl-2 family proteins Bcl-2, Bcl-xl and Bcl-w, disrupts the interaction between Bcl-2/Bcl-xl /Bcl-w and pro-apoptotic proteins such as Bim, Bad and Bak, which trigger the caspases-initiated cell death pathway to induce apoptosis.
References:
1. Tse et al., ABT-263: A Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008, 68, 3421-3428.
2. Shoemaker et al., Activity of the Bcl-2 Family Inhibitor ABT-263 in a Panel of Small Cell Lung Cancer Xenograft Models. Clin. Cancer Res. 2008, 14, 3268-3277
- SAR407899
Catalog No.:BCC5593
CAS No.:923359-38-0
- Nilotinib monohydrochloride monohydrate
Catalog No.:BCC1801
CAS No.:923288-90-8
- Opicapone
Catalog No.:BCC6545
CAS No.:923287-50-7
- SAR407899 hydrochloride
Catalog No.:BCC5592
CAS No.:923262-96-8
- Refametinib R enantiomer
Catalog No.:BCC4055
CAS No.:923032-38-6
- Refametinib
Catalog No.:BCC4276
CAS No.:923032-37-5
- 3-O-Methyl-3-methoxymaxterone
Catalog No.:BCC8639
CAS No.:92282-70-7
- Sanggenon N
Catalog No.:BCN4846
CAS No.:92280-12-1
- (±)-MDMA hydrochloride
Catalog No.:BCC5965
CAS No.:92279-84-0
- Daphnilongeridine
Catalog No.:BCN4460
CAS No.:922522-15-4
- Syringin pentaacetate
Catalog No.:BCN4459
CAS No.:92233-55-1
- 5,6-Desmethylenedioxy-5-methoxyaglalactone
Catalog No.:BCN7639
CAS No.:922169-96-8
- GSK962040
Catalog No.:BCC4163
CAS No.:923565-21-3
- GSK962040 hydrochloride
Catalog No.:BCC4152
CAS No.:923565-22-4
- Vaniprevir
Catalog No.:BCC2030
CAS No.:923590-37-8
- Simeprevir
Catalog No.:BCC1949
CAS No.:923604-59-5
- 3-Acetoxy-4-cadinen-8-one
Catalog No.:BCN4461
CAS No.:923950-05-4
- DUBs-IN-3
Catalog No.:BCC5258
CAS No.:924296-17-3
- DUBs-IN-1
Catalog No.:BCC5256
CAS No.:924296-18-4
- DUBs-IN-2
Catalog No.:BCC5257
CAS No.:924296-19-5
- HBX 41108
Catalog No.:BCC6137
CAS No.:924296-39-9
- AdipoRon
Catalog No.:BCC4756
CAS No.:924416-43-3
- T 5601640
Catalog No.:BCC5617
CAS No.:924473-59-6
- AZD-5597
Catalog No.:BCC6453
CAS No.:924641-59-8
Data on the DNA damaging and mutagenic potential of the BH3-mimetics ABT-263/Navitoclax and TW-37.[Pubmed:26958630]
Data Brief. 2016 Jan 16;6:710-4.
Unfortunately, the mutagenic activities of chemotherapy and radiotherapy can provoke development of therapy-induced malignancies in cancer survivors. Non-mutagenic anti-cancer therapies may be less likely to trigger subsequent malignant neoplasms. Here we present data regarding the DNA damaging and mutagenic potential of two drugs that antagonize proteins within the Bcl-2 family: ABT-263/Navitoclax and TW-37. Our data reveal that concentrations of these agents that stimulated Bax/Bak-dependent signaling provoked little DNA damage and failed to trigger mutations in surviving cells. The data supplied in this article is related to the research work entitled "Inhibition of Bcl-2 or IAP proteins does not provoke mutations in surviving cells" [1].
Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with irinotecan: results of an open-label, phase 1 study.[Pubmed:26429709]
Cancer Chemother Pharmacol. 2015 Nov;76(5):1041-9.
PURPOSE: The oral Bcl-2 inhibitor navitoclax demonstrated activity in solid and hematologic malignancies as monotherapy and in combination with other cytotoxic agents in preclinical and early clinical studies. We evaluated the safety, pharmacokinetics (PK), and antitumor activity of navitoclax plus irinotecan. METHODS: In this multicenter, open-label, phase 1 dose escalation study, adults with advanced solid tumors received navitoclax (starting dose 150 mg/day) in combination with 1 of 2 irinotecan schedules during a 21-day cycle: a once-every-3-week regimen (Q3W 180, 250, or 350 mg/m(2)) or a once-weekly regimen (QW 75 or 100 mg/m(2)). Enrollment occurred until a maximum tolerated dose (MTD) and/or recommended phase 2 dose (RPTD) was reached. RESULTS: All patients (Q3W, n = 14; QW, n = 17) were evaluable for safety, PK, and efficacy. The most common adverse event in both groups was diarrhea (Q3W 92.9 %; QW 76.5 %), which was the most frequent grade 3/grade 4 adverse event (Q3W 42.9 %; QW 29.4 %). The study was amended to exclude 4 UGT1A1*28 7/7 homozygous patients due to frequent irinotecan-related grade 3/grade 4 diarrhea and/or febrile neutropenia. No apparent PK interactions between navitoclax and irinotecan were observed. The MTD of the combination was exceeded in the Q3W group at the lowest dose administered. In the QW group, the MTD and RPTD for navitoclax were 150 mg when combined with irinotecan 75 mg/m(2). One patient in each group achieved a partial response. CONCLUSION: The RPTD of navitoclax in combination with irinotecan 75 mg/m(2) QW during a 21-day cycle was 150 mg in these heavily pretreated patients.
Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with erlotinib in patients with advanced solid tumors.[Pubmed:26420235]
Cancer Chemother Pharmacol. 2015 Nov;76(5):1025-32.
PURPOSE: Navitoclax (ABT-263), a novel, oral Bcl-2 inhibitor, enhances the antitumor effects of chemotherapy in vitro by lowering the apoptotic threshold. This phase I study (NCT01009073) evaluated the safety, pharmacokinetics, and preliminary antitumor activity of navitoclax combined with erlotinib in patients with advanced solid tumors. PATIENTS AND METHODS: An open-label dose escalation study included an arm evaluating navitoclax combined with erlotinib, which included a dose escalation cohort and a planned safety expansion cohort. Patients with documented cancers for whom erlotinib therapy was appropriate received erlotinib 150 mg orally once daily plus navitoclax 150 mg orally once daily, with navitoclax dose escalation via a continuous reassessment method model. RESULTS: Eleven patients were enrolled, including six patients with nonsmall cell lung cancer. Dose-limiting toxicities, most commonly diarrhea, were observed in 4 patients. Navitoclax dosing remained at 150 mg/day because the maximum tolerated dose was exceeded at this starting dose. The planned dose escalation did not occur; no recommended phase II dose (RPTD) was identified, and there was no safety expansion cohort. The most common treatment-related adverse events were diarrhea, nausea, vomiting, and decreased appetite. Pharmacokinetic analysis showed no apparent interactions between co-administered navitoclax and erlotinib. No objective responses were observed; the disease control rate was 27 % (95 % CI, 6-61 %). CONCLUSION: At the erlotinib and navitoclax doses administered, RPTD was not reached, but the safety profile of the combination was consistent with data from monotherapy studies. There were no apparent pharmacokinetic interactions between erlotinib and navitoclax. Three patients had stable disease.
Combination with vorinostat overcomes ABT-263 (navitoclax) resistance of small cell lung cancer.[Pubmed:26575826]
Cancer Biol Ther. 2016;17(1):27-35.
Small cell lung cancer (SCLC) is an aggressive tumor type with high mortality. One promising approach for SCLC treatment would be to utilize agents targeting molecular abnormalities regulating resistance to apoptosis. BH3 mimetic antagonists, such as ABT-737 and its orally available derivative ABT-263 (Navitoclax) have been developed to block the function of pro-survival BCL-2 family members. The sensitivity of SCLC to these drugs varies over a broad range in vitro and in clinical trials. We have previously shown that the expression of Noxa, a BH3-only pro-apoptotic BCL-2 family protein, is a critical determinant of sensitivity to ABT-737. Thus, pharmacological up-regulation of Noxa could enhance cell death induced by the BH3 mimetics. We find that the combination of ABT-263 and a HDAC inhibitor, vorinostat, efficiently induces apoptosis in a variety of SCLC cell lines, including ABT-263 resistant cell lines. Cell death induced by combined treatment is Noxa- and/or BIM-dependent in some cell lines but in others appears to be mediated by down-regulation of BCL-XL and release of BAK from BCL-XL and MCL-1. These results suggest that combination of HDAC inhibitors and BCL-2 inhibitors could be an alternative and effective regimen for SCLC treatment.


