OpicaponeCAS# 923287-50-7 |
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- Dihydroeponemycin
Catalog No.:BCC3596
CAS No.:126463-64-7
- MG-132
Catalog No.:BCC1227
CAS No.:133407-82-6
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

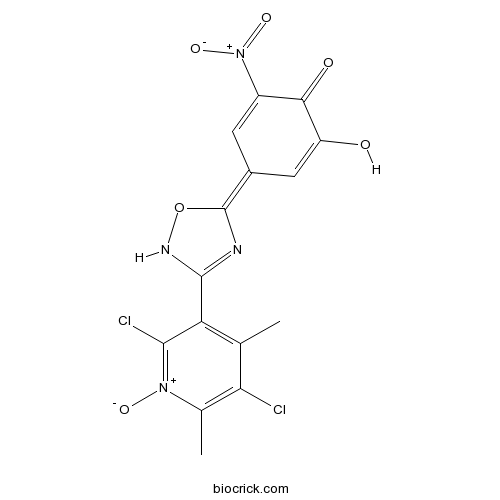
| Cas No. | 923287-50-7 | SDF | Download SDF |
| PubChem ID | 15602694 | Appearance | Powder |
| Formula | C15H10Cl2N4O6 | M.Wt | 413.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BIA 9-1067 | ||
| Solubility | DMSO : 100 mg/mL (242.03 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | (4E)-4-[3-(2,5-dichloro-4,6-dimethyl-1-oxidopyridin-1-ium-3-yl)-2H-1,2,4-oxadiazol-5-ylidene]-2-hydroxy-6-nitrocyclohexa-2,5-dien-1-one | ||
| SMILES | CC1=C(C(=[N+](C(=C1Cl)C)[O-])Cl)C2=NC(=C3C=C(C(=O)C(=C3)O)[N+](=O)[O-])ON2 | ||
| Standard InChIKey | HVGGGVAREUUJQV-VIZOYTHASA-N | ||
| Standard InChI | InChI=1S/C15H10Cl2N4O6/c1-5-10(13(17)20(24)6(2)11(5)16)14-18-15(27-19-14)7-3-8(21(25)26)12(23)9(22)4-7/h3-4,22H,1-2H3,(H,18,19)/b15-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Opicapone Dilution Calculator

Opicapone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4203 mL | 12.1016 mL | 24.2031 mL | 48.4062 mL | 60.5078 mL |
| 5 mM | 0.4841 mL | 2.4203 mL | 4.8406 mL | 9.6812 mL | 12.1016 mL |
| 10 mM | 0.242 mL | 1.2102 mL | 2.4203 mL | 4.8406 mL | 6.0508 mL |
| 50 mM | 0.0484 mL | 0.242 mL | 0.4841 mL | 0.9681 mL | 1.2102 mL |
| 100 mM | 0.0242 mL | 0.121 mL | 0.242 mL | 0.4841 mL | 0.6051 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Opicapone is an available catechol-O-methyltransferase (COMT) inhibitor. Opicapone decreases the ATP content of the cells with IC50 values of 98 μM.
In Vitro:Opicapone has a prolonged inhibitory effect on peripheral COMT, which extends the bioavailability of levodopa, without inducing toxicity. Opicapone decreases the ATP content of the cells with IC50 values of 98 μM. Incubation of human primary hepatocytes for 24 h with increasing concentrations of Tolcapone, entacapone or Opicapone resulted in a concentration-dependent decrease in the mitochondrial membrane potential of the cells, evaluated by the ratio JC-1 aggregates over JC-1 monomer (ratio λex 544 λem 590 over λex 485 λem 538). Opicapone decreases the mitochondrial membrane potential of the cells with IC50 of 181 μM[1].
In Vivo:Opicapone inhibits rat peripheral COMT with ED50 values below 1.4 mg/kg up to 6 h post-administration. The effect is sustained over the first 8 h and by 24 h COMT had not returned to control values. A single administration of Opicapone resulted in increased and sustained plasma levodopa levels with a concomitant reduction in 3-O-methyldopa from 2 h up to 24 h post-administration, while Tolcapone produced significant effects only at 2 h post-administration. The effects of Opicapone on brain catecholamines after levodopa administration are sustained up to 24 h post-administration. Opicapone is also the least potent compound in decreasing both the mitochondrial membrane potential and the ATP content in human primary hepatocytes after a 24 h incubation period[1].
References:
[1]. Bonifácio MJ, et al. Pharmacological profile of Opicapone, a third-generation nitrocatechol catechol-O-methyl transferase inhibitor, in the rat. Br J Pharmacol. 2015 Apr;172(7):1739-52.
- SAR407899 hydrochloride
Catalog No.:BCC5592
CAS No.:923262-96-8
- Refametinib R enantiomer
Catalog No.:BCC4055
CAS No.:923032-38-6
- Refametinib
Catalog No.:BCC4276
CAS No.:923032-37-5
- 3-O-Methyl-3-methoxymaxterone
Catalog No.:BCC8639
CAS No.:92282-70-7
- Sanggenon N
Catalog No.:BCN4846
CAS No.:92280-12-1
- (±)-MDMA hydrochloride
Catalog No.:BCC5965
CAS No.:92279-84-0
- Daphnilongeridine
Catalog No.:BCN4460
CAS No.:922522-15-4
- Syringin pentaacetate
Catalog No.:BCN4459
CAS No.:92233-55-1
- 5,6-Desmethylenedioxy-5-methoxyaglalactone
Catalog No.:BCN7639
CAS No.:922169-96-8
- GlcNAcstatin
Catalog No.:BCC5334
CAS No.:922163-64-2
- SCH 529074
Catalog No.:BCC6131
CAS No.:922150-11-6
- 12-O-Tiglylphorbol-13-isobutyrate
Catalog No.:BCN2512
CAS No.:92214-54-5
- Nilotinib monohydrochloride monohydrate
Catalog No.:BCC1801
CAS No.:923288-90-8
- SAR407899
Catalog No.:BCC5593
CAS No.:923359-38-0
- ABT-263 (Navitoclax)
Catalog No.:BCC1272
CAS No.:923564-51-6
- GSK962040
Catalog No.:BCC4163
CAS No.:923565-21-3
- GSK962040 hydrochloride
Catalog No.:BCC4152
CAS No.:923565-22-4
- Vaniprevir
Catalog No.:BCC2030
CAS No.:923590-37-8
- Simeprevir
Catalog No.:BCC1949
CAS No.:923604-59-5
- 3-Acetoxy-4-cadinen-8-one
Catalog No.:BCN4461
CAS No.:923950-05-4
- DUBs-IN-3
Catalog No.:BCC5258
CAS No.:924296-17-3
- DUBs-IN-1
Catalog No.:BCC5256
CAS No.:924296-18-4
- DUBs-IN-2
Catalog No.:BCC5257
CAS No.:924296-19-5
- HBX 41108
Catalog No.:BCC6137
CAS No.:924296-39-9
Pharmacokinetics of opicapone, a third-generation COMT inhibitor, after single and multiple oral administration: A comparative study in the rat.[Pubmed:28322896]
Toxicol Appl Pharmacol. 2017 May 15;323:9-15.
Opicapone is a novel potent, reversible and purely peripheral catechol-O-methyltransferase inhibitor that has been developed to be used as an adjunct to levodopa/aromatic L-amino acid decarboxylase inhibitor therapy for Parkinson's disease. Thus, this study aimed to compare the plasma pharmacokinetics of Opicapone and its active metabolite (BIA 9-1079) after the administration of single and multiple oral doses to rats. Wistar rats (n=8 per group) were orally treated with single (30, 60 or 90mg/kg) or multiple (30mg/kg once-daily for seven consecutive days) oral doses of Opicapone. Blood samples were collected up to 24h post-dosing through a cannula introduced in the tail vein of rats. After quantifying Opicapone and BIA 9-1079 in plasma, a non-compartmental pharmacokinetic analysis was performed. Opicapone was quickly absorbed (time to reach the maximum plasma concentrationOpicapone increased approximately in a dose-proportional manner after single-dosing within the studied dose range (30-90mg/kg). Opicapone and BIA 9-1079 showed a relatively short plasma elimination half-life (1.58-4.50h) and a small systemic accumulation after multiple-dosing. Hence, no pharmacokinetic concerns are expected when Opicapone is administered with a once-daily dosing regimen.
Opicapone as Adjunct to Levodopa Therapy in Patients With Parkinson Disease and Motor Fluctuations: A Randomized Clinical Trial.[Pubmed:28027332]
JAMA Neurol. 2017 Feb 1;74(2):197-206.
Importance: Catechol O-methyltransferase (COMT) inhibitors are an established treatment for end-of-dose motor fluctuations associated with levodopa therapy in patients with Parkinson disease (PD). Current COMT inhibitors carry a high risk for toxic effects to hepatic cells or show moderate improvement. Opicapone was designed to be effective without the adverse effects. Objective: To evaluate the efficacy and safety of 25- and 50-mg/d dosages of Opicapone compared with placebo as adjunct to levodopa therapy in patients with PD experiencing end-of-dose motor fluctuations. Design: This phase 3 international, multicenter outpatient study evaluated a 25- and a 50-mg/d dosage of Opicapone in a randomized, double-blind, 14- to 15-week, placebo-controlled clinical trial, followed by a 1-year open-label phase during which all patients received active treatment with Opicapone. Patients with PD who experienced signs of end-of-dose deterioration and had a mean total awake off-time (state of akinesia or decreased mobility) of at least 1.5 hours, not including morning akinesia, were enrolled. Data were collected from March 18, 2011, through June 25, 2013. Data from the evaluable population were analyzed from July 31, 2013, to July 31, 2014. Main Outcomes and Measures: The primary efficacy outcome of the double-blind phase was the change from baseline in absolute off-time vs placebo based on patient diaries. The open-label phase focused on maintenance of treatment effect in off-time. Results: A total of 427 patients (258 men [60.4%] and 169 women [39.6%]; mean [SD] age, 63.1 [8.8] years) were randomized to a 25-mg/d (n = 129) or a 50-mg/d (n = 154) dosage of Opicapone or to placebo (n = 144). Of these, 376 patients completed the double-blind phase and entered the open-label phase, of whom 286 completed 1 year of open-label treatment. At the end of the double-blind phase, the least squares mean change (SE) in off-time was -64.5 (14.4) minutes for the placebo group, -101.7 (14.9) minutes for the 25-mg/d Opicapone group, and -118.8 (13.8) minutes for the 50-mg/d Opicapone group. The adjusted treatment difference vs placebo was significant for the 50-mg/d Opicapone group (treatment effect, -54.3 [95% CI, -96.2 to -12.4] minutes; P = .008), but not for the 25-mg/d Opicapone group (treatment effect, -37.2 [95% CI, -80.8 to 6.4] minutes; P = .11). The off-time reduction was sustained throughout the open-label phase (-126.3 minutes at 1-year open-label end point). The most common adverse events in the Opicapone vs placebo groups were dyskinesia, constipation, and dry mouth. Fifty-one patients (11.9%) discontinued from the study during the double-blind phase. Conclusions and Relevance: Treatment with a 50-mg once-daily dose of Opicapone was associated with a significant reduction in mean daily off-time in levodopa-treated patients with PD and motor fluctuations, and this effect is maintained for at least 1 year. Opicapone was safe and well tolerated. Trial Registration: clinicaltrials.gov Identifier: NCT01227655.
Spotlight on opicapone as an adjunct to levodopa in Parkinson's disease: design, development and potential place in therapy.[Pubmed:28123288]
Drug Des Devel Ther. 2017 Jan 9;11:143-151.
Parkinson's disease (PD) is a progressive, chronic, neurodegenerative disease characterized by rigidity, tremor, bradykinesia and postural instability secondary to dopaminergic deficit in the nigrostriatal system. Currently, disease-modifying therapies are not available, and levodopa (LD) treatment remains the gold standard for controlling motor and nonmotor symptoms of the disease. LD is extensively and rapidly metabolized by peripheral enzymes, namely, aromatic amino acid decarboxylase and catechol-O-methyltransferase (COMT). To increase the bioavailability of LD, COMT inhibitors are frequently used in clinical settings. Opicapone is a novel COMT inhibitor that has been recently approved by the European Medicines Agency as an adjunctive therapy to combinations of LD and aromatic amino acid decarboxylase inhibitor in adult PD patients with end-of-dose motor fluctuations. We aimed to review the biochemical properties of Opicapone, summarize its preclinical and clinical trials and discuss its future potential role in the treatment of PD.
Opicapone for the treatment of Parkinson's disease.[Pubmed:28234566]
Expert Opin Pharmacother. 2017 Mar;18(4):445-453.
INTRODUCTION: Parkinson's disease (PD) is a progressive neurodegenerative disease. The currently available treatment options only have a symptomatic effect. With disease progression almost all antiparkinsonian pharmacological classes are tried, but the gold standard of pharmacological management is still L-dopa. Various strategies can be used to raise the dopaminergic tone. Catechol-O-methyltransferase (COMT) inhibitors attain this goal by decreasing L-dopa peripheral metabolism. Areas covered: Opicapone (Ongentys(R)) is a new COMT inhibitor developed to fulfill the need for more potent, safer and longer acting COMT inhibitors. This review puts into context Opicapone's indications, its chemical and preclinical data, the pharmacodynamics and pharmacokinetic characteristics, and the efficacy and safety results delivered by clinical trials. Expert opinion: Opicapone is an efficacious COMT inhibitor. Its proprieties make it adequate for a once-a-day oral dose regimen. It has proved to reduce the off-time and to increase the on-time without troublesome dyskinesias in PD patients with motor fluctuations. The reported adverse events suggest an overall safe and well-tolerated profile. The most common adverse events were dyskinesia, and there were no issues of concern for hepatotoxicity, severe diarrhoea or chromaturia. Further evidence is still needed to conclude how it compares with other drugs for the treatment of motor fluctuations.


