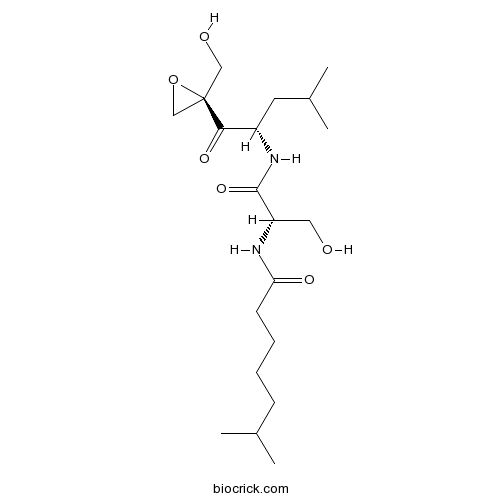
DihydroeponemycinProteasome inhibitor,antitumor reagent,eponemycin ddrivative CAS# 126463-64-7 |
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- CEP-18770
Catalog No.:BCC2093
CAS No.:847499-27-8
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 126463-64-7 | SDF | Download SDF |
| PubChem ID | 10092400 | Appearance | Powder |
| Formula | C20H36N2O6 | M.Wt | 400.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >15.6mg/mL in DMSO | ||
| Chemical Name | N-[(2S)-3-hydroxy-1-[[(2S)-1-[(2R)-2-(hydroxymethyl)oxiran-2-yl]-4-methyl-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]-6-methylheptanamide | ||
| SMILES | CC(C)CCCCC(=O)NC(CO)C(=O)NC(CC(C)C)C(=O)C1(CO1)CO | ||
| Standard InChIKey | IUDBVFIQSSOIDB-TWOQFEAHSA-N | ||
| Standard InChI | InChI=1S/C20H36N2O6/c1-13(2)7-5-6-8-17(25)21-16(10-23)19(27)22-15(9-14(3)4)18(26)20(11-24)12-28-20/h13-16,23-24H,5-12H2,1-4H3,(H,21,25)(H,22,27)/t15-,16-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dihydroeponemycin is an inhibitor of proteasome and antitumor reagent. | |||||
| Targets | Proteasome | |||||

Dihydroeponemycin Dilution Calculator

Dihydroeponemycin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4968 mL | 12.4841 mL | 24.9682 mL | 49.9363 mL | 62.4204 mL |
| 5 mM | 0.4994 mL | 2.4968 mL | 4.9936 mL | 9.9873 mL | 12.4841 mL |
| 10 mM | 0.2497 mL | 1.2484 mL | 2.4968 mL | 4.9936 mL | 6.242 mL |
| 50 mM | 0.0499 mL | 0.2497 mL | 0.4994 mL | 0.9987 mL | 1.2484 mL |
| 100 mM | 0.025 mL | 0.1248 mL | 0.2497 mL | 0.4994 mL | 0.6242 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description:IC50: 100 nm (antiproliferative IC50) [1]
The proteasome is a member of the growing N-terminal nucleophile (Ntn) hydrolase family, whose aminoterminal side chains act as nucleophiles and free amino groups as proton acceptors. Proteasome inhibitors have been increasingly used to help define the role of the proteasome in cell biology. Dihydroeponemycin labels the catalytic threonine residues of the immunoproteasome subunits LMP2 and LMP7 and the constitutive proteasome subunit X, while epoxomicin covalently modifies the N-terminal catalytic threonine residues of the constitutive proteasome (X and Z) and immunoproteasome (LMP7 and MECL1) subunits.
In vitro: Previoius study reports that dihydroeponemycin, an analogue of the antitumor and antiangiogenic natural product eponemycin, selectively targets the 20S proteasome. Dihydroeponemycin covalently modifies a subset of catalytic proteasomal subunits, binding preferentially to the IFN-g-inducible subunits LMP2 and LMP7. Moreover, the three major peptidolytic activities of the proteasome are inhibited by dihydroeponemycin at different rates. In addition, dihydroeponemycin-mediated proteasome inhibition induces a spindle-like cellular morphological change and apoptosis. These results validate the proteasome as a target for antitumor pharmacological intervention and are relevant for the design of novel chemotherapeutic strategies [2].
In vivo: Recent study in the mouse model of dihydrosterptomycin shows that hair cells are targeted because aminoglycosides enter outer hair cells through large mechanically gated channels in the mechanosensory hair boundles. The aminoglycoside molecules then block the channels through which they entered. When this occurs, the aminoglycoside goes through the channel and effectively traps itself in the hair cell [3].
Clinical trial: Dihydroeponemycin is currently in the preclinical development and non clinical trial is ongoing.
References:
[1] Kim KB, Myung J, Sin N, Crews CM. Proteasome inhibition by the natural products epoxomicin and dihydroeponemycin: insights into specificity and potency. Bioorg Med Chem Lett. 1999;9(23):3335-40.
[2] Meng L, Kwok BH, Sin N, Crews CM. Eponemycin exerts its antitumor effect through the inhibition of proteasome function. Cancer Res. 1999;59(12):2798-801.
[3] Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol. 2005;567(Pt 2):505-21.
- (1S,2R)-1-Amino-2-indanol
Catalog No.:BCC8384
CAS No.:126456-43-7
- (R)-CPP
Catalog No.:BCC6581
CAS No.:126453-07-4
- 6-Ethoxygeniposide
Catalog No.:BCN7043
CAS No.:1264496-61-8
- Pyrrolam B
Catalog No.:BCN1986
CAS No.:126424-77-9
- Pyrrolam A
Catalog No.:BCN2040
CAS No.:126424-76-8
- VTP-27999 Hydrochloride
Catalog No.:BCC2050
CAS No.:1264191-73-2
- SR 12813
Catalog No.:BCC7530
CAS No.:126411-39-0
- Colistin Sulfate
Catalog No.:BCC4653
CAS No.:1264-72-8
- Nortropinyl cinnamate
Catalog No.:BCN1891
CAS No.:126394-79-4
- Pinobanksin 3-O-propanoate
Catalog No.:BCN7737
CAS No.:126394-70-5
- CDK9 inhibitor 2
Catalog No.:BCC1466
CAS No.:1263369-28-3
- 7-Chlorokynurenic acid sodium salt
Catalog No.:BCC7757
CAS No.:1263094-00-3
- Metasequirin D
Catalog No.:BCN4781
CAS No.:1264694-96-3
- Mupirocin
Catalog No.:BCC5558
CAS No.:12650-69-0
- CH5183284 (Debio-1347)
Catalog No.:BCC5649
CAS No.:1265229-25-1
- Ciclesonide
Catalog No.:BCC5234
CAS No.:126544-47-6
- 8,14-Epoxyergosta-4,22-diene-3,6-dione
Catalog No.:BCN1591
CAS No.:1265908-20-0
- S1RA hydrochloride
Catalog No.:BCC4190
CAS No.:1265917-14-3
- Cerberidol
Catalog No.:BCN6142
CAS No.:126594-64-7
- Cyclocerberidol
Catalog No.:BCN6143
CAS No.:126594-66-9
- Vallesamine N-oxide
Catalog No.:BCN6144
CAS No.:126594-73-8
- Trilobinine
Catalog No.:BCN7927
CAS No.:126595-92-4
- A 887826
Catalog No.:BCC7898
CAS No.:1266212-81-0
- 16-Epinormacusine B
Catalog No.:BCN4030
CAS No.:126640-98-0
Proteasome inhibition by the natural products epoxomicin and dihydroeponemycin: insights into specificity and potency.[Pubmed:10612595]
Bioorg Med Chem Lett. 1999 Dec 6;9(23):3335-40.
While two structurally related epoxyketone-containing antitumor natural products, epoxomicin and eponemycin, share the proteasome as a common intracellular target, they differ in their antiproliferative activity, proteasome subunit binding specificity, and rates of proteasome inhibition. As a first step towards understanding such differences and developing novel proteasome subunit-specific inhibitors, we report here the synthesis and characterization of epoxomicin/Dihydroeponemycin chimerae.


