CH5183284 (Debio-1347)CAS# 1265229-25-1 |
- AZD4547
Catalog No.:BCC3711
CAS No.:1035270-39-3
- SKLB610
Catalog No.:BCC3647
CAS No.:1125780-41-7
- AP26113
Catalog No.:BCC1069
CAS No.:1197958-12-5
- PD 173074
Catalog No.:BCC3662
CAS No.:219580-11-7
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
- Nintedanib (BIBF 1120)
Catalog No.:BCC3661
CAS No.:656247-17-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1265229-25-1 | SDF | Download SDF |
| PubChem ID | 66555680 | Appearance | Powder |
| Formula | C20H16N6O | M.Wt | 356.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Debio 1347 | ||
| Solubility | DMSO : 25 mg/mL (70.15 mM; ultrasonic and warming and heat to 50°C) | ||
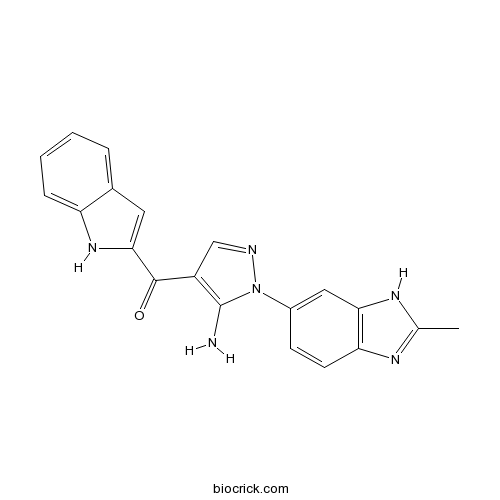
| Chemical Name | [5-amino-1-(2-methyl-3H-benzimidazol-5-yl)pyrazol-4-yl]-(1H-indol-2-yl)methanone | ||
| SMILES | CC1=NC2=C(N1)C=C(C=C2)N3C(=C(C=N3)C(=O)C4=CC5=CC=CC=C5N4)N | ||
| Standard InChIKey | BEMNJULZEQTDJY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H16N6O/c1-11-23-16-7-6-13(9-17(16)24-11)26-20(21)14(10-22-26)19(27)18-8-12-4-2-3-5-15(12)25-18/h2-10,25H,21H2,1H3,(H,23,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CH5183284 is an orally available and selective FGFR inhibitor with IC50s of 9.3, 7.6, and 22 nM for FGFR1, FGFR2, FGFR3, and FGFR4, respectively.In Vitro:CH5183284 is well balanced in cellular antiproliferative activity against SNU-16 and stability in human liver microsome. The selectivity of 8 to inhibit FGFR over KDR is suggested to be caused by the difference in the interaction with M535 in FGFR1 and L889 in KDR[1]. The IC50 of CH5183284/Debio 1347 is 29 nM for FGF-dependent proliferation and 780 nM for VEGF-dependent proliferation[2].In Vivo:CH5183284 treatment shows a dose-dependent tumor regression (tumor growth inhibition (TGI)=106% at 30 mg/kg and 147% at 100 mg/kg) without apparent body weight loss. CH5183284 treatment also shows significant in vivo efficacy in xenograft mice models with FGFR genetic alterations, such as KG1 (leukemia, FGFR1OP-FGFR1 fusion), MFE280 (endometrial cancer, FGFR2 S252W mutation), UM-UC-14 (bladder cancer, FGFR3 S249C mutation), and RT112/84 (bladder cancer, FGFR3-TACC3 fusion)[1]. References: | |||||

CH5183284 (Debio-1347) Dilution Calculator

CH5183284 (Debio-1347) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.806 mL | 14.03 mL | 28.0599 mL | 56.1199 mL | 70.1498 mL |
| 5 mM | 0.5612 mL | 2.806 mL | 5.612 mL | 11.224 mL | 14.03 mL |
| 10 mM | 0.2806 mL | 1.403 mL | 2.806 mL | 5.612 mL | 7.015 mL |
| 50 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1224 mL | 1.403 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2806 mL | 0.5612 mL | 0.7015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CH5183284 is a selective and orally available FGFR inhibitor with IC50 of 9.3 nM, 7.6 nM, 22 nM, and 290 nM for FGFR1, FGFR2, FGFR3, and FGFR4, respectively. Phase 1.
- Mupirocin
Catalog No.:BCC5558
CAS No.:12650-69-0
- Metasequirin D
Catalog No.:BCN4781
CAS No.:1264694-96-3
- Dihydroeponemycin
Catalog No.:BCC3596
CAS No.:126463-64-7
- (1S,2R)-1-Amino-2-indanol
Catalog No.:BCC8384
CAS No.:126456-43-7
- (R)-CPP
Catalog No.:BCC6581
CAS No.:126453-07-4
- 6-Ethoxygeniposide
Catalog No.:BCN7043
CAS No.:1264496-61-8
- Pyrrolam B
Catalog No.:BCN1986
CAS No.:126424-77-9
- Pyrrolam A
Catalog No.:BCN2040
CAS No.:126424-76-8
- VTP-27999 Hydrochloride
Catalog No.:BCC2050
CAS No.:1264191-73-2
- SR 12813
Catalog No.:BCC7530
CAS No.:126411-39-0
- Colistin Sulfate
Catalog No.:BCC4653
CAS No.:1264-72-8
- Nortropinyl cinnamate
Catalog No.:BCN1891
CAS No.:126394-79-4
- Ciclesonide
Catalog No.:BCC5234
CAS No.:126544-47-6
- 8,14-Epoxyergosta-4,22-diene-3,6-dione
Catalog No.:BCN1591
CAS No.:1265908-20-0
- S1RA hydrochloride
Catalog No.:BCC4190
CAS No.:1265917-14-3
- Cerberidol
Catalog No.:BCN6142
CAS No.:126594-64-7
- Cyclocerberidol
Catalog No.:BCN6143
CAS No.:126594-66-9
- Vallesamine N-oxide
Catalog No.:BCN6144
CAS No.:126594-73-8
- Trilobinine
Catalog No.:BCN7927
CAS No.:126595-92-4
- A 887826
Catalog No.:BCC7898
CAS No.:1266212-81-0
- 16-Epinormacusine B
Catalog No.:BCN4030
CAS No.:126640-98-0
- KT 5823
Catalog No.:BCC7006
CAS No.:126643-37-6
- UTPγS trisodium salt
Catalog No.:BCC7624
CAS No.:1266569-94-1
- Isoaltenuene
Catalog No.:BCN7313
CAS No.:126671-80-5
Discovery of [5-Amino-1-(2-methyl-3H-benzimidazol-5-yl)pyrazol-4-yl]-(1H-indol-2-yl)methanone (CH5183284/Debio 1347), An Orally Available and Selective Fibroblast Growth Factor Receptor (FGFR) Inhibitor.[Pubmed:27933954]
J Med Chem. 2016 Dec 8;59(23):10586-10600.
The fibroblast growth factor receptor (FGFR) family of receptor tyrosine kinases regulates multiple biological processes, such as cell proliferation, migration, apoptosis, and differentiation. Various genetic alterations that drive activation of the receptors and the pathway are associated with tumor growth and survival; therefore, the FGFR family represents an attractive therapeutic target for treating cancer. Here, we report the discovery and the pharmacological profiles of 8 (CH5183284/Debio 1347), an orally available and selective inhibitor of FGFR1, FGFR2, and FGFR3. The chemical modifications, which were guided by 3D-modeling analyses of the inhibitor and FGFRs, led to identifying an inhibitor that is selective to FGFR1, FGFR2, and FGFR3. In in vitro studies and xenograft models in mice, 8 shows antitumor activity against cancer cell lines that harbor genetically altered FGFRs. These results support the potential therapeutic use of 8 as a new anticancer agent.
ERK Signal Suppression and Sensitivity to CH5183284/Debio 1347, a Selective FGFR Inhibitor.[Pubmed:26438159]
Mol Cancer Ther. 2015 Dec;14(12):2831-9.
Drugs that target specific gene alterations have proven beneficial in the treatment of cancer. Because cancer cells have multiple resistance mechanisms, it is important to understand the downstream pathways of the target genes and monitor the pharmacodynamic markers associated with therapeutic efficacy. We performed a transcriptome analysis to characterize the response of various cancer cell lines to a selective fibroblast growth factor receptor (FGFR) inhibitor (CH5183284/Debio 1347), a mitogen-activated protein kinase kinase (MEK) inhibitor, or a phosphoinositide 3-kinase (PI3K) inhibitor. FGFR and MEK inhibition produced similar expression patterns, and the extracellular signal-regulated kinase (ERK) gene signature was altered in several FGFR inhibitor-sensitive cell lines. Consistent with these findings, CH5183284/Debio 1347 suppressed phospho-ERK in every tested FGFR inhibitor-sensitive cell line. Because the mitogen-activated protein kinase (MAPK) pathway functions downstream of FGFR, we searched for a pharmacodynamic marker of FGFR inhibitor efficacy in a collection of cell lines with the ERK signature and identified dual-specificity phosphatase 6 (DUSP6) as a candidate marker. Although a MEK inhibitor suppressed the MAPK pathway, most FGFR inhibitor-sensitive cell lines are insensitive to MEK inhibitors and we found potent feedback activation of several pathways via FGFR. We therefore suggest that FGFR inhibitors exert their effect by suppressing ERK signaling without feedback activation. In addition, DUSP6 may be a pharmacodynamic marker of FGFR inhibitor efficacy in FGFR-addicted cancers.
The fibroblast growth factor receptor genetic status as a potential predictor of the sensitivity to CH5183284/Debio 1347, a novel selective FGFR inhibitor.[Pubmed:25169980]
Mol Cancer Ther. 2014 Nov;13(11):2547-58.
The FGF receptors (FGFR) are tyrosine kinases that are constitutively activated in a subset of tumors by genetic alterations such as gene amplifications, point mutations, or chromosomal translocations/rearrangements. Recently, small-molecule inhibitors that can inhibit the FGFR family as well as the VEGF receptor (VEGFR) or platelet-derived growth factor receptor (PDGFR) family displayed clinical benefits in cohorts of patients with FGFR genetic alterations. However, to achieve more potent and prolonged activity in such populations, a selective FGFR inhibitor is still needed. Here, we report the identification of CH5183284/Debio 1347, a selective and orally available FGFR1, FGFR2, and FGFR3 inhibitor that has a unique chemical scaffold. By interacting with unique residues in the ATP-binding site of FGFR1, FGFR2, or FGFR3, CH5183284/Debio 1347 selectively inhibits FGFR1, FGFR2, and FGFR3 but does not inhibit kinase insert domain receptor (KDR) or other kinases. Consistent with its high selectivity for FGFR enzymes, CH5183284/Debio 1347 displayed preferential antitumor activity against cancer cells with various FGFR genetic alterations in a panel of 327 cancer cell lines and in xenograft models. Because of its unique binding mode, CH5183284/Debio 1347 can inhibit FGFR2 harboring one type of the gatekeeper mutation that causes resistance to other FGFR inhibitors and block FGFR2 V564F-driven tumor growth. CH5183284/Debio 1347 is under clinical investigation for the treatment of patients harboring FGFR genetic alterations.


