8,14-Epoxyergosta-4,22-diene-3,6-dioneCAS# 1265908-20-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1265908-20-0 | SDF | Download SDF |
| PubChem ID | 49870999 | Appearance | Powder |
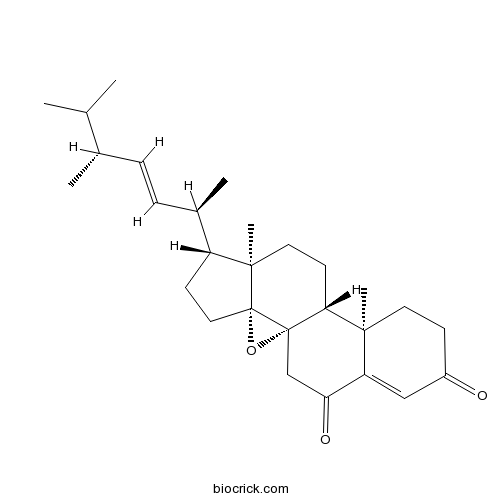
| Formula | C28H40O3 | M.Wt | 424.62 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,3R,6R,7R,10R,11R)-6-[(E,2R,5R)-5,6-dimethylhept-3-en-2-yl]-7,11-dimethyl-2-oxapentacyclo[8.8.0.01,3.03,7.011,16]octadec-15-ene-14,17-dione | ||
| SMILES | CC(C)C(C)C=CC(C)C1CCC23C1(CCC4C2(O3)CC(=O)C5=CC(=O)CCC45C)C | ||
| Standard InChIKey | DJVSRKXHTYPLOV-ZNUAOLICSA-N | ||
| Standard InChI | InChI=1S/C28H40O3/c1-17(2)18(3)7-8-19(4)21-10-14-28-26(21,6)13-11-24-25(5)12-9-20(29)15-22(25)23(30)16-27(24,28)31-28/h7-8,15,17-19,21,24H,9-14,16H2,1-6H3/b8-7+/t18-,19+,21+,24+,25-,26+,27-,28+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (22E,24R)-8,14-epoxyergosta-4,22-diene-3,6-dione has antitumor activity, it may be disarming oncogenic pathways via direct modulation of the epigenetic machinery. |
| In vitro | Chemical Constituents of Papulaspora immersa, an Endophyte from Smallanthus sonchifolius (Asteraceae), and Their Cytotoxic Activity.[Reference: WebLink]Chemistry & Biodiversity, 2010, 7(12):2941-2950.Papulaspora immersa H. H. Hotson was isolated from roots and leaves of Smallanthus sonchifolius (Poepp. and Endl.) H. Rob. (Asteraceae), traditionally known as Yacon.
The anti-promyelocytic leukemia mode of action of two endophytic secondary metabolites unveiled by a proteomic approach.[Reference: WebLink]Planta Medica, 2014, 80(06):473-481.
|

8,14-Epoxyergosta-4,22-diene-3,6-dione Dilution Calculator

8,14-Epoxyergosta-4,22-diene-3,6-dione Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.355 mL | 11.7752 mL | 23.5505 mL | 47.1009 mL | 58.8762 mL |
| 5 mM | 0.471 mL | 2.355 mL | 4.7101 mL | 9.4202 mL | 11.7752 mL |
| 10 mM | 0.2355 mL | 1.1775 mL | 2.355 mL | 4.7101 mL | 5.8876 mL |
| 50 mM | 0.0471 mL | 0.2355 mL | 0.471 mL | 0.942 mL | 1.1775 mL |
| 100 mM | 0.0236 mL | 0.1178 mL | 0.2355 mL | 0.471 mL | 0.5888 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ciclesonide
Catalog No.:BCC5234
CAS No.:126544-47-6
- CH5183284 (Debio-1347)
Catalog No.:BCC5649
CAS No.:1265229-25-1
- Mupirocin
Catalog No.:BCC5558
CAS No.:12650-69-0
- Metasequirin D
Catalog No.:BCN4781
CAS No.:1264694-96-3
- Dihydroeponemycin
Catalog No.:BCC3596
CAS No.:126463-64-7
- (1S,2R)-1-Amino-2-indanol
Catalog No.:BCC8384
CAS No.:126456-43-7
- (R)-CPP
Catalog No.:BCC6581
CAS No.:126453-07-4
- 6-Ethoxygeniposide
Catalog No.:BCN7043
CAS No.:1264496-61-8
- Pyrrolam B
Catalog No.:BCN1986
CAS No.:126424-77-9
- Pyrrolam A
Catalog No.:BCN2040
CAS No.:126424-76-8
- VTP-27999 Hydrochloride
Catalog No.:BCC2050
CAS No.:1264191-73-2
- SR 12813
Catalog No.:BCC7530
CAS No.:126411-39-0
- S1RA hydrochloride
Catalog No.:BCC4190
CAS No.:1265917-14-3
- Cerberidol
Catalog No.:BCN6142
CAS No.:126594-64-7
- Cyclocerberidol
Catalog No.:BCN6143
CAS No.:126594-66-9
- Vallesamine N-oxide
Catalog No.:BCN6144
CAS No.:126594-73-8
- Trilobinine
Catalog No.:BCN7927
CAS No.:126595-92-4
- A 887826
Catalog No.:BCC7898
CAS No.:1266212-81-0
- 16-Epinormacusine B
Catalog No.:BCN4030
CAS No.:126640-98-0
- KT 5823
Catalog No.:BCC7006
CAS No.:126643-37-6
- UTPγS trisodium salt
Catalog No.:BCC7624
CAS No.:1266569-94-1
- Isoaltenuene
Catalog No.:BCN7313
CAS No.:126671-80-5
- Gancaonin G
Catalog No.:BCN6837
CAS No.:126716-34-5
- Gancaonin I
Catalog No.:BCN7144
CAS No.:126716-36-7
The anti-promyelocytic leukemia mode of action of two endophytic secondary metabolites unveiled by a proteomic approach.[Pubmed:24710897]
Planta Med. 2014 Apr;80(6):473-81.
As a result of a program to find antitumor compounds of endophytes from medicinal Asteraceae, the steroid (22E,24R)-8,14-Epoxyergosta-4,22-diene-3,6-dione (a) and the diterpene aphidicolin (b) were isolated from the filamentous fungi Papulaspora immersa and Nigrospora sphaerica, respectively, and exhibited strong cytotoxicity against HL-60 cells. A proteomic approach was used in an attempt to identify the drugs' molecular targets and their respective antiproliferative mode of action. Results suggested that the (a) growth inhibition effect occurs by G2/M cell cycle arrest via reduction of tubulin alpha and beta isomers and 14-3-3 protein gamma expression, followed by a decrease of apoptotic and inflammatory proteins, culminating in mitochondrial oxidative damage that triggered autophagy-associated cell death. Moreover, the decrease observed in the expression levels of several types of histones indicated that (a) might be disarming oncogenic pathways via direct modulation of the epigenetic machinery. Effects on cell cycle progression and induction of apoptosis caused by (b) were confirmed. In addition, protein expression profiles also revealed that aphidicolin is able to influence microtubule dynamics, modulate proteasome activator complex expression, and control the inflammatory cascade through overexpression of thymosin beta 4, RhoGDI2, and 14-3-3 proteins. Transmission electron micrographs of (b)-treated cells unveiled dose-dependent morphological characteristics of autophagy- or oncosis-like cell death.
Chemical constituents of Papulaspora immersa, an endophyte from Smallanthus sonchifolius (Asteraceae), and their cytotoxic activity.[Pubmed:21162007]
Chem Biodivers. 2010 Dec;7(12):2941-50.
Papulaspora immersa H. H. Hotson was isolated from roots and leaves of Smallanthus sonchifolius (Poepp. and Endl.) H. Rob. (Asteraceae), traditionally known as Yacon. The fungus was cultured in rice, and, from the AcOEt fraction, 14 compounds were isolated. Among them, (22E,24R)-8,14-Epoxyergosta-4,22-diene-3,6-dione (4), 2,3-epoxy-1,2,3,4-tetrahydronaphthalene-c-1,c-4,8-triol (10), and the chromone papulasporin (13) were new secondary metabolites. The spectral data of the known natural products were compared with the literature data, and their structures were established as the (24R)-stigmast-4-en-3-one (1), 24-methylenecycloartan-3beta-ol (2), (22E,24R)-ergosta-4,6,8(14),22-tetraen-3-one (3), (-)-(3R,4R)-4-hydroxymellein (5), (-)-(3R)-5-hydroxymellein (6), 6,8-dihydroxy-3-methylisocoumarin (7), (-)-(4S)-4,8-dihydroxy-alpha-tetralone (8), naphthalene-1,8-diol (9), 6,7,8-trihydroxy-3-methylisocoumarin (11), 7-hydroxy-2,5-dimethylchromone (12), and tyrosol (14). Compound 4 showed the highest cytotoxic activity against the human tumor cell lines MDA-MB435 (melanoma), HCT-8 (colon), SF295 (glioblastoma), and HL-60 (promyelocytic leukemia), with IC(5)(0) values of 3.3, 14.7, 5.0 and 1.6 muM, respectively. Strong synergistic effects were also observed with compound 5 and some of the isolated steroidal compounds.


