S1RA hydrochlorideσ1R antagonist CAS# 1265917-14-3 |
- Sodium Channel inhibitor 1
Catalog No.:BCC1959
CAS No.:1198117-23-5
- Nav1.7 inhibitor
Catalog No.:BCC4191
CAS No.:1355631-24-1
- Riluzole
Catalog No.:BCC3849
CAS No.:1744-22-5
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
- Dibucaine (Cinchocaine) HCl
Catalog No.:BCC3760
CAS No.:61-12-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1265917-14-3 | SDF | Download SDF |
| PubChem ID | 50914801 | Appearance | Powder |
| Formula | C20H24ClN3O2 | M.Wt | 373.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | E-52862 hydrochloride | ||
| Solubility | DMSO : ≥ 57 mg/mL (152.46 mM) *"≥" means soluble, but saturation unknown. | ||
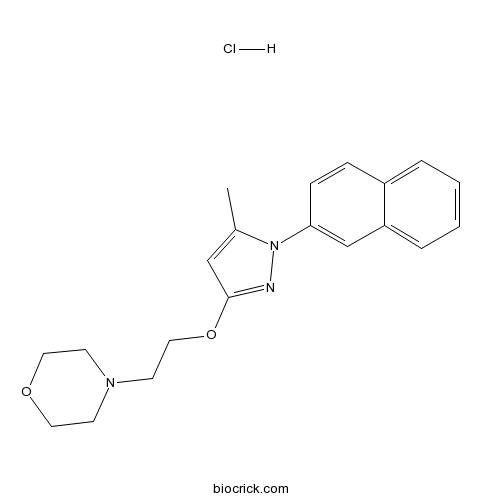
| Chemical Name | 4-[2-(5-methyl-1-naphthalen-2-ylpyrazol-3-yl)oxyethyl]morpholine;hydrochloride | ||
| SMILES | CC1=CC(=NN1C2=CC3=CC=CC=C3C=C2)OCCN4CCOCC4.Cl | ||
| Standard InChIKey | SHRYQZBTQDMGLZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H23N3O2.ClH/c1-16-14-20(25-13-10-22-8-11-24-12-9-22)21-23(16)19-7-6-17-4-2-3-5-18(17)15-19;/h2-7,14-15H,8-13H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | S1RA Hcl(E-52862 Hcl) is a potent and selective sigma-1 receptor(σ1R, Ki=17 nM) antagonist, showed good selectivity against σ2R (Ki > 1000 nM).
IC50 value: 17 nM (Ki) [1]
Target: σ1R antagonist
in vitro: S1RA behaved as a highly selective σ1 receptor antagonist. It showed high affinity for human (Ki= 17 nM) and guinea pig (Ki= 23.5 nM) σ1 receptors but no significant affinity for the σ2 receptors (Ki > 1000 nM for guinea pig and rat σ2 receptors). Moderate affinity (Ki= 328 nM) and antagonistic activity, with very low potency (IC50= 4700 nM) was found at the human 5-HT2B receptor. S1RA showed no significant affinity (Ki > 1 μM or % inhibition at 1 μM < 50%) for other additional 170 targets (receptors, transporters, ion channels and enzymes) [2].
in vivo: Control (non-operated) and nerve-injured mice received a single or repeated (twice daily for 12 days) i.p. administration of S1RA at 25 mg·kg?1, the same dose used for the assessment of behavioural hypersensitivity in the chronic treatment study. Acute treatment was given on day 12 post-surgery and repeated treatment with S1RA started the day of surgery, as in the behavioural studies [2]. Intrathecal pre-treatment with idazoxan prevented the systemic S1RA antinociceptive effect, suggesting that the S1RA antinociception depends on the activation of spinal α2 -adrenoceptors which, in turn, could induce an inhibition of formalin-evoked glutamate release. When administered locally, intrathecal S1RA inhibited only the flinching behavior, whereas intracerebroventricularly or intraplantarly injected also attenuated the lifting/licking behavior [3]. References: | |||||

S1RA hydrochloride Dilution Calculator

S1RA hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6747 mL | 13.3733 mL | 26.7465 mL | 53.4931 mL | 66.8664 mL |
| 5 mM | 0.5349 mL | 2.6747 mL | 5.3493 mL | 10.6986 mL | 13.3733 mL |
| 10 mM | 0.2675 mL | 1.3373 mL | 2.6747 mL | 5.3493 mL | 6.6866 mL |
| 50 mM | 0.0535 mL | 0.2675 mL | 0.5349 mL | 1.0699 mL | 1.3373 mL |
| 100 mM | 0.0267 mL | 0.1337 mL | 0.2675 mL | 0.5349 mL | 0.6687 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
S1RA hydrochloride is a potent and selective antagonist of σ1 receptor (σ1R) with Ki value of 17nM [1].
S1RA is the first σ1 receptor antagonist with potent antinociceptive activities in various pain models. In the binding assay, S1RA shows good affinity to human σ1 receptor transfected in HEK293 membranes with Ki value of 17nM. The Ki value for guinea pig brain membrane σ1 receptor is higher than 1μM. S1RA also shows no significant affinity to another 170 molecular targets including receptors, ion channels and enzymes [1, 2].
In the mouse tests, S1RA exhibits potent analgesic effects on capsaicin-induced mechanical hypersensitivity and formalin-induced pain. Besides that, S1RA inhibits both mechanical allodynia and thermal hypersensitivity with ED50 values of 23.4mg/kg and 18.8mg/kg in the partial sciatic nerve ligation model in mice [1].
References:
[1] Díaz J L, Cuberes R, Berrocal J, et al. Synthesis and Biological Evaluation of the 1-Arylpyrazole Class of σ1 Receptor Antagonists: Identification of 4-{2-[5-Methyl-1-(naphthalen-2-yl)-1 H-pyrazol-3-yloxy] ethyl} morpholine (S1RA, E-52862). Journal of medicinal chemistry, 2012, 55(19): 8211-8224.
[2] Wunsch B. The σ1 Receptor Antagonist S1RA Is a Promising Candidate for the Treatment of Neurogenic PainJ. Journal of medicinal chemistry, 2012, 55(19): 8209-8210.
- 8,14-Epoxyergosta-4,22-diene-3,6-dione
Catalog No.:BCN1591
CAS No.:1265908-20-0
- Ciclesonide
Catalog No.:BCC5234
CAS No.:126544-47-6
- CH5183284 (Debio-1347)
Catalog No.:BCC5649
CAS No.:1265229-25-1
- Mupirocin
Catalog No.:BCC5558
CAS No.:12650-69-0
- Metasequirin D
Catalog No.:BCN4781
CAS No.:1264694-96-3
- Dihydroeponemycin
Catalog No.:BCC3596
CAS No.:126463-64-7
- (1S,2R)-1-Amino-2-indanol
Catalog No.:BCC8384
CAS No.:126456-43-7
- (R)-CPP
Catalog No.:BCC6581
CAS No.:126453-07-4
- 6-Ethoxygeniposide
Catalog No.:BCN7043
CAS No.:1264496-61-8
- Pyrrolam B
Catalog No.:BCN1986
CAS No.:126424-77-9
- Pyrrolam A
Catalog No.:BCN2040
CAS No.:126424-76-8
- VTP-27999 Hydrochloride
Catalog No.:BCC2050
CAS No.:1264191-73-2
- Cerberidol
Catalog No.:BCN6142
CAS No.:126594-64-7
- Cyclocerberidol
Catalog No.:BCN6143
CAS No.:126594-66-9
- Vallesamine N-oxide
Catalog No.:BCN6144
CAS No.:126594-73-8
- Trilobinine
Catalog No.:BCN7927
CAS No.:126595-92-4
- A 887826
Catalog No.:BCC7898
CAS No.:1266212-81-0
- 16-Epinormacusine B
Catalog No.:BCN4030
CAS No.:126640-98-0
- KT 5823
Catalog No.:BCC7006
CAS No.:126643-37-6
- UTPγS trisodium salt
Catalog No.:BCC7624
CAS No.:1266569-94-1
- Isoaltenuene
Catalog No.:BCN7313
CAS No.:126671-80-5
- Gancaonin G
Catalog No.:BCN6837
CAS No.:126716-34-5
- Gancaonin I
Catalog No.:BCN7144
CAS No.:126716-36-7
- Tilifodiolide
Catalog No.:BCN6145
CAS No.:126724-95-6
Modulation of peripheral mu-opioid analgesia by sigma1 receptors.[Pubmed:24155346]
J Pharmacol Exp Ther. 2014 Jan;348(1):32-45.
We evaluated the effects of sigma1-receptor inhibition on mu-opioid-induced mechanical antinociception and constipation. sigma1-Knockout mice exhibited marked mechanical antinociception in response to several mu-opioid analgesics (fentanyl, oxycodone, morphine, buprenorphine, and tramadol) at systemic (subcutaneous) doses that were inactive in wild-type mice and even unmasked the antinociceptive effects of the peripheral mu-opioid agonist loperamide. Likewise, systemic (subcutaneous) or local (intraplantar) treatment of wild-type mice with the selective sigma1 antagonists BD-1063 [1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine dihydrochloride] or S1RA [4-[2-[[5-methyl-1-(2-naphthalenyl)1H-pyrazol-3-yl]oxy]ethyl] morpholine hydrochloride] potentiated mu-opioid antinociception; these effects were fully reversed by the sigma1 agonist PRE-084 [2-(4-morpholinethyl)1-phenylcyclohexanecarboxylate) hydrochloride], showing the selectivity of the pharmacological approach. The mu-opioid antinociception potentiated by sigma1 inhibition (by sigma1-receptor knockout or sigma1-pharmacological antagonism) was more sensitive to the peripherally restricted opioid antagonist naloxone methiodide than opioid antinociception under normal conditions, indicating a key role for peripheral opioid receptors in the enhanced antinociception. Direct interaction between the opioid drugs and sigma1 receptor cannot account for our results, since the former lacked affinity for sigma1 receptors (labeled with [(3)H](+)-pentazocine). A peripheral role for sigma1 receptors was also supported by their higher density (Western blot results) in peripheral nervous tissue (dorsal root ganglia) than in several central areas involved in opioid antinociception (dorsal spinal cord, basolateral amygdala, periaqueductal gray, and rostroventral medulla). In contrast to its effects on nociception, sigma1-receptor inhibition did not alter fentanyl- or loperamide-induced constipation, a peripherally mediated nonanalgesic opioid effect. Therefore, sigma1-receptor inhibition may be used as a systemic or local adjuvant to enhance peripheral mu-opioid analgesia without affecting opioid-induced constipation.


