Sodium Channel inhibitor 1Voltage-gated sodium channel for pain treatment CAS# 1198117-23-5 |
- S0859
Catalog No.:BCC1914
CAS No.:1019331-10-2
- Nav1.7 inhibitor
Catalog No.:BCC4191
CAS No.:1355631-24-1
- Riluzole
Catalog No.:BCC3849
CAS No.:1744-22-5
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
- Dibucaine (Cinchocaine) HCl
Catalog No.:BCC3760
CAS No.:61-12-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

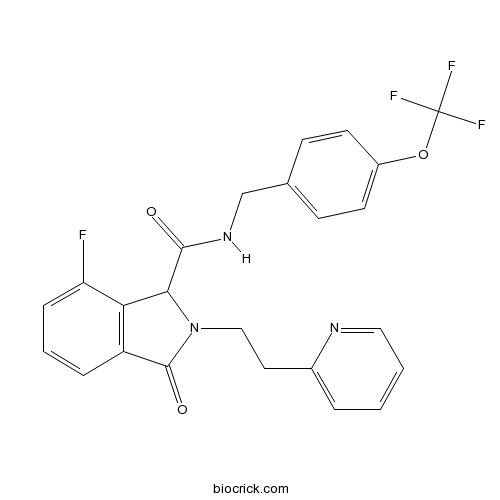
| Cas No. | 1198117-23-5 | SDF | Download SDF |
| PubChem ID | 44545403 | Appearance | Powder |
| Formula | C24H19F4N3O3 | M.Wt | 473.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | 7-fluoro-3-oxo-2-(2-pyridin-2-ylethyl)-N-[[4-(trifluoromethoxy)phenyl]methyl]-1H-isoindole-1-carboxamide | ||
| SMILES | C1=CC=NC(=C1)CCN2C(C3=C(C2=O)C=CC=C3F)C(=O)NCC4=CC=C(C=C4)OC(F)(F)F | ||
| Standard InChIKey | GRXUKFHZQDPFAI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H19F4N3O3/c25-19-6-3-5-18-20(19)21(31(23(18)33)13-11-16-4-1-2-12-29-16)22(32)30-14-15-7-9-17(10-8-15)34-24(26,27)28/h1-10,12,21H,11,13-14H2,(H,30,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sodium Channel inhibitor 1 Dilution Calculator

Sodium Channel inhibitor 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1123 mL | 10.5614 mL | 21.1229 mL | 42.2458 mL | 52.8072 mL |
| 5 mM | 0.4225 mL | 2.1123 mL | 4.2246 mL | 8.4492 mL | 10.5614 mL |
| 10 mM | 0.2112 mL | 1.0561 mL | 2.1123 mL | 4.2246 mL | 5.2807 mL |
| 50 mM | 0.0422 mL | 0.2112 mL | 0.4225 mL | 0.8449 mL | 1.0561 mL |
| 100 mM | 0.0211 mL | 0.1056 mL | 0.2112 mL | 0.4225 mL | 0.5281 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 0.16 uM ( Na v1.7, V hold-90mV); 0.41 uM (Na v1.7, V hold-90mV) [1] Sodium Channel inhibitor1, one of 3-Oxoisoindoline-1-carboxamides, is a novel and selective voltage-gated sodium channel for pain treatment. Sodium Channel inhibitor1 demonstrated concentration-dependent e?cacy in preclinical behavioral pain models.
- Mirin
Catalog No.:BCC5986
CAS No.:1198097-97-0
- AP26113
Catalog No.:BCC1069
CAS No.:1197958-12-5
- 3-Furfuryl 2-pyrrolecarboxylate
Catalog No.:BCN6086
CAS No.:119767-00-9
- 29-Norlanosta-8,24-diene-1alpha,2alpha,3beta-triol
Catalog No.:BCN7984
CAS No.:119765-92-3
- Baohuoside VI
Catalog No.:BCC8129
CAS No.:119760-73-5
- Tautomycetin
Catalog No.:BCC7320
CAS No.:119757-73-2
- Schizanthine M
Catalog No.:BCN1939
CAS No.:119736-78-6
- Schizanthine G
Catalog No.:BCN1938
CAS No.:119736-74-2
- SB 206553 hydrochloride
Catalog No.:BCC7143
CAS No.:1197334-04-5
- SDZ 205-557 hydrochloride
Catalog No.:BCC7246
CAS No.:1197334-02-3
- Sazetidine A dihydrochloride
Catalog No.:BCC7468
CAS No.:1197329-42-2
- TGR5 Receptor Agonist
Catalog No.:BCC4195
CAS No.:1197300-24-5
- Cerdulatinib (PRT062070)
Catalog No.:BCC8068
CAS No.:1198300-79-6
- Coptisine sulfate
Catalog No.:BCN2286
CAS No.:1198398-71-8
- Phyperunolide E
Catalog No.:BCN7292
CAS No.:1198400-52-0
- Esomeprazole magnesium salt
Catalog No.:BCC5430
CAS No.:1198768-91-0
- Posaconazole hydrate
Catalog No.:BCC4234
CAS No.:1198769-38-8
- CGRP 8-37 (human)
Catalog No.:BCC5724
CAS No.:119911-68-1
- 7-O-Methylmorroniside
Catalog No.:BCN7293
CAS No.:119943-46-3
- Peroxy Orange 1
Catalog No.:BCC6336
CAS No.:1199576-10-7
- 1,2-Didehydrotanshinone IIA
Catalog No.:BCN3143
CAS No.:119963-50-7
- INT-777
Catalog No.:BCC5390
CAS No.:1199796-29-6
- Unedone
Catalog No.:BCN6759
CAS No.:1199815-09-2
- Sulfuretin
Catalog No.:BCN4725
CAS No.:120-05-8
Discovery of triazolopyridine GS-458967, a late sodium current inhibitor (Late INai) of the cardiac NaV 1.5 channel with improved efficacy and potency relative to ranolazine.[Pubmed:27080178]
Bioorg Med Chem Lett. 2016 Jul 1;26(13):3202-3206.
We started with a medium throughput screen of heterocyclic compounds without basic amine groups to avoid hERG and beta-blocker activity and identified [1,2,4]triazolo[4,3-a]pyridine as an early lead. Optimization of substituents for Late INa current inhibition and lack of Peak INa inhibition led to the discovery of 4h (GS-458967) with improved anti-arrhythmic activity relative to ranolazine. Unfortunately, 4h demonstrated use dependent block across the sodium isoforms including the central and peripheral nervous system isoforms that is consistent with its low therapeutic index (approximately 5-fold in rat, 3-fold in dog). Compound 4h represents our initial foray into a 2nd generation Late INa inhibitor program and is an important proof-of-concept compound. We will provide additional reports on addressing the CNS challenge in a follow-up communication.
Compound-specific effects of mutations at Val787 in DII-S6 of Nav 1.4 sodium channels on the action of sodium channel inhibitor insecticides.[Pubmed:22983119]
Neurotoxicology. 2012 Oct;33(5):1381-9.
Sodium channel inhibitor (SCI) insecticides are hypothesized to inhibit voltage-gated sodium channels by binding selectively to the slow-inactivated state. Replacement of valine at position 787 in the S6 segment of homology domain II of the rat Na(v)1.4 sodium channel by lysine (V787K) enchances slow inactivation of this channel whereas replacement by alanine or cysteine (V787A and V787C) inhibits slow inactivation. To test the hypothesis that SCI insecticides bind selectively to the slow-inactivated state, we constructed mutated Na(v)1.4/V787A, Na(v)1.4/V787C, and Na(v)1.4/V787K cDNAs, expressed wildtype and mutated channels with the auxiliary beta1 subunit in Xenopus oocytes, and used the two-electrode voltage clamp technique to examine the effects of these mutations on channel inhibition by four SCI insecticides (indoxacarb, its bioactivated metabolite DCJW, metaflumizone, and RH3421). Mutations at Val787 affected SCI insecticide sensitivity in a manner that was independent of mutation-induced changes in slow inactivation gating. Sensitivity to inhibition by 10 muM indoxacarb was significantly increased in all three mutated channels, whereas sensitivity to inhibition by 10 muM metaflumizone was significantly reduced in Na(v)1.4/V787A channels and completely abolished in Na(v)1.4/V787K channels. The effects of Val787 mutations on metaflumizone were correlated with the hydrophobicity of the substituted amino acid rather than the extent of slow inactivation. None of the mutations at Val787 significantly affected the sensitivity to inhibition by DCJW or RH3421. These results demonstrate that the impact of mutations at Val787 on sodium channel inhibition by SCI insecticides depend on the specific insecticide examined and is independent of mutation-induced changes in slow inactivation gating. We propose that Val787 may be a unique determinant of metaflumizone binding.


