MirinMRN-ATM pathway inhibitor CAS# 1198097-97-0 |
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- (R,R)-Formoterol
Catalog No.:BCC1293
CAS No.:67346-49-0
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- Medetomidine
Catalog No.:BCC1736
CAS No.:86347-14-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1198097-97-0 | SDF | Download SDF |
| PubChem ID | 1206243 | Appearance | Powder |
| Formula | C10H8N2O2S | M.Wt | 220.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 31 mg/mL (140.75 mM) *"≥" means soluble, but saturation unknown. | ||
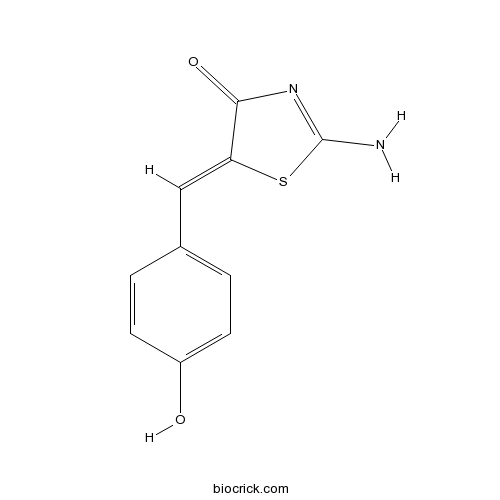
| Chemical Name | (5Z)-2-amino-5-[(4-hydroxyphenyl)methylidene]-1,3-thiazol-4-one | ||
| SMILES | C1=CC(=CC=C1C=C2C(=O)N=C(S2)N)O | ||
| Standard InChIKey | YBHQCJILTOVLHD-YVMONPNESA-N | ||
| Standard InChI | InChI=1S/C10H8N2O2S/c11-10-12-9(14)8(15-10)5-6-1-3-7(13)4-2-6/h1-5,13H,(H2,11,12,14)/b8-5- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mre11-Rad50-Nbs1 (MRN)-ATM pathway inhibitor that blocks the 3' and 5' exonuclease activity associated with Mre11. Prevents ATM activation in response to double strand breaks (IC50 = 12 μM) and induces G2 cell cycle arrest. Also blocks homology-directed repair in vitro. |

Mirin Dilution Calculator

Mirin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5403 mL | 22.7015 mL | 45.403 mL | 90.8059 mL | 113.5074 mL |
| 5 mM | 0.9081 mL | 4.5403 mL | 9.0806 mL | 18.1612 mL | 22.7015 mL |
| 10 mM | 0.454 mL | 2.2701 mL | 4.5403 mL | 9.0806 mL | 11.3507 mL |
| 50 mM | 0.0908 mL | 0.454 mL | 0.9081 mL | 1.8161 mL | 2.2701 mL |
| 100 mM | 0.0454 mL | 0.227 mL | 0.454 mL | 0.9081 mL | 1.1351 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Mirin is a small-molecule inhibitor of MRN (Mre11, Rad50, and Nbs1) complex. Target: in vitro: Mirin was shown to block Mre11 exonuclease activity and MRN-dependent ATM activation, and to inhibit the ionizing radiation-induced G2/M checkpoint and homologous recombination in mammalian cells. Mirin inhibition of ATM activation is independent of Mre11 nuclease inhibition since the M(HN)RN and M(HL/DV)RN mutant complexes are inhibited equivalently to the wild-type enzyme despite the fact that they are nuclease-deficient. The effects of Mirin have been ascribed to its effects on Mre11 nuclease activity, but as we show here, Mirin is inhibitory of MRN function independent of the nuclease activity of the complex. [2] Addition of the Mre11 inhibitor Mirin to egg extracts and mammalian cells reduces RCC1 association with mitotic chromosomes. HeLa cells expressing Histone H2B-EYFP were treated with Mirin (25 or 50 μM), and mitotic progression was immediately monitored using live cell imaging. Although Mirin-treated cells were able to congress chromosomes to the metaphase plate with similar kinetics as untreated cells, a significant fraction of Mirin-treated cells paused for extended periods of time in a metaphase-like stage without anaphase onset . [1]
References:
[1]. Rozier L, et al. The MRN-CtIP pathway is required for metaphase chromosome alignment. Mol Cell. 2013 Mar 28;49(6):1097-107.
[2]. Lee JH, et al. Ataxia telangiectasia-mutated (ATM) kinase activity is regulated by ATP-driven conformational changes in the Mre11/Rad50/Nbs1 (MRN) complex. J Biol Chem. 2013 May 3;288(18):12840-51.
[3]. Garner KM, et al. Corrected structure of Mirin, a small-molecule inhibitor of the Mre11-Rad50-Nbs1 complex. Nat Chem Biol. 2009 Mar;5(3):129-30.
- AP26113
Catalog No.:BCC1069
CAS No.:1197958-12-5
- 3-Furfuryl 2-pyrrolecarboxylate
Catalog No.:BCN6086
CAS No.:119767-00-9
- 29-Norlanosta-8,24-diene-1alpha,2alpha,3beta-triol
Catalog No.:BCN7984
CAS No.:119765-92-3
- Baohuoside VI
Catalog No.:BCC8129
CAS No.:119760-73-5
- Tautomycetin
Catalog No.:BCC7320
CAS No.:119757-73-2
- Schizanthine M
Catalog No.:BCN1939
CAS No.:119736-78-6
- Schizanthine G
Catalog No.:BCN1938
CAS No.:119736-74-2
- SB 206553 hydrochloride
Catalog No.:BCC7143
CAS No.:1197334-04-5
- SDZ 205-557 hydrochloride
Catalog No.:BCC7246
CAS No.:1197334-02-3
- Sazetidine A dihydrochloride
Catalog No.:BCC7468
CAS No.:1197329-42-2
- TGR5 Receptor Agonist
Catalog No.:BCC4195
CAS No.:1197300-24-5
- Baohuoside VII
Catalog No.:BCN2889
CAS No.:119730-89-1
- Sodium Channel inhibitor 1
Catalog No.:BCC1959
CAS No.:1198117-23-5
- Cerdulatinib (PRT062070)
Catalog No.:BCC8068
CAS No.:1198300-79-6
- Coptisine sulfate
Catalog No.:BCN2286
CAS No.:1198398-71-8
- Phyperunolide E
Catalog No.:BCN7292
CAS No.:1198400-52-0
- Esomeprazole magnesium salt
Catalog No.:BCC5430
CAS No.:1198768-91-0
- Posaconazole hydrate
Catalog No.:BCC4234
CAS No.:1198769-38-8
- CGRP 8-37 (human)
Catalog No.:BCC5724
CAS No.:119911-68-1
- 7-O-Methylmorroniside
Catalog No.:BCN7293
CAS No.:119943-46-3
- Peroxy Orange 1
Catalog No.:BCC6336
CAS No.:1199576-10-7
- 1,2-Didehydrotanshinone IIA
Catalog No.:BCN3143
CAS No.:119963-50-7
- INT-777
Catalog No.:BCC5390
CAS No.:1199796-29-6
- Unedone
Catalog No.:BCN6759
CAS No.:1199815-09-2
Formation of ethyl ferulate by rice koji enzyme in sake and mirin mash conditions.[Pubmed:23597918]
J Biosci Bioeng. 2013 Aug;116(2):209-13.
Formation mechanism of ethyl ferulate (EF) in sake and Mirin mash conditions was investigated to understand EF level control in the manufacturing process. Rice koji formed EF from ferulic acid (FA) and ethanol and decomposed EF to FA. This did not occur in sake yeast and chemical esterification was rare. Esterification of FA and hydrolysis of EF by rice koji might be due to feruloyl esterase(s). The rice koji enzyme showed normal Michaelis-Menten kinetics for FA in ethyl esterification and for EF in hydrolysis, but not for ethanol in the esterification reaction. Substrate specificity of the rice koji enzyme for hydroxycinnamic acids suggested that the main enzyme involved might be similar to type A feruloyl esterase. We studied the rice koji enzyme properties, short-term digestion of steamed rice grains with exogenous ethanol and small scale Mirin making with pH adjustment. Our results suggested differences in the esterification and hydrolysis properties of the enzyme, in particular, different pH dependencies and different behaviors under high ethanol conditions; these factors might cause the differing EF levels in sake and Mirin mashes.
Mirin: identifying microRNA regulatory modules in protein-protein interaction networks.[Pubmed:24794934]
Bioinformatics. 2014 Sep 1;30(17):2527-8.
UNLABELLED: Exploring microRNA (miRNA) regulations and protein-protein interactions could reveal the molecular mechanisms responsible for complex biological processes. Mirin is a web-based application suitable for identifying functional modules from protein-protein interaction networks regulated by aberrant miRNAs under user-defined biological conditions such as cancers. The analysis involves combining miRNA regulations, protein-protein interactions between target genes, as well as mRNA and miRNA expression profiles provided by users. Mirin has successfully uncovered oncomirs and their regulatory networks in various cancers, such as gastric and breast cancer. AVAILABILITY AND IMPLEMENTATION: Mirin is freely available at http://Mirin.ym.edu.tw/. SUPPLEMENTARY INFORMATION: Supplementary data are available at Bioinformatics online.
Aroma compounds in Japanese sweet rice wine (Mirin) screened by aroma extract dilution analysis (AEDA).[Pubmed:25391446]
Biosci Biotechnol Biochem. 2015;79(3):484-7.
Thirty-nine key aroma compounds were newly identified or tentatively identified in the aroma concentrate of Japanese sweet rice wine (Mirin) by an aroma extract dilution analysis technique based on the 68 detected peaks. Among them, 3-(methylthio)propanal, 3-hydroxy-4,5-dimethyl-2(5H)-furanone, 3-methylbutanoic acid, 2-methylbutanoic acid, and 2-methoxy-4-vinylphenol were detected with the highest FD factors in this study.
A forward chemical genetic screen reveals an inhibitor of the Mre11-Rad50-Nbs1 complex.[Pubmed:18176557]
Nat Chem Biol. 2008 Feb;4(2):119-25.
The MRN (Mre11-Rad50-Nbs1)-ATM (ataxia-telangiectasia mutated) pathway is essential for sensing and signaling from DNA double-strand breaks. The MRN complex acts as a DNA damage sensor, maintains genome stability during DNA replication, promotes homology-dependent DNA repair and activates ATM. MRN is essential for cell viability, which has limited functional studies of the complex. Small-molecule inhibitors of MRN could circumvent this experimental limitation and could also be used as cellular radio- and chemosensitization compounds. Using cell-free systems that recapitulate faithfully the MRN-ATM signaling pathway, we designed a forward chemical genetic screen to identify inhibitors of the pathway, and we isolated 6-(4-hydroxyphenyl)-2-thioxo-2,3-dihydro-4(1H)-pyrimidinone (Mirin, 1) as an inhibitor of MRN. Mirin prevents MRN-dependent activation of ATM without affecting ATM protein kinase activity, and it inhibits Mre11-associated exonuclease activity. Consistent with its ability to target the MRN complex, Mirin abolishes the G2/M checkpoint and homology-dependent repair in mammalian cells.


