TautomycetinSelective PP1 inhibitor CAS# 119757-73-2 |
- Quercetin
Catalog No.:BCN6049
CAS No.:117-39-5
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- MEK inhibitor
Catalog No.:BCC1738
CAS No.:334951-92-7
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 119757-73-2 | SDF | Download SDF |
| PubChem ID | 6439037 | Appearance | Powder |
| Formula | C33H50O10 | M.Wt | 606.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO (supplied pre-dissolved in DMSO, 10mg/ml) | ||
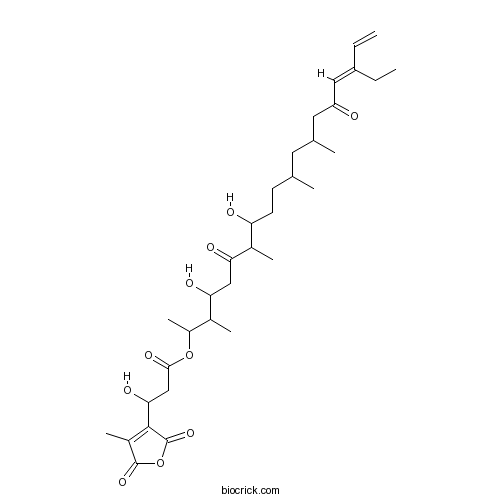
| Chemical Name | [(16E)-17-ethyl-4,8-dihydroxy-3,7,11,13-tetramethyl-6,15-dioxononadeca-16,18-dien-2-yl] 3-hydroxy-3-(4-methyl-2,5-dioxofuran-3-yl)propanoate | ||
| SMILES | CCC(=CC(=O)CC(C)CC(C)CCC(C(C)C(=O)CC(C(C)C(C)OC(=O)CC(C1=C(C(=O)OC1=O)C)O)O)O)C=C | ||
| Standard InChIKey | VAIBGAONSFVVKI-IWIPYMOSSA-N | ||
| Standard InChI | InChI=1S/C33H50O10/c1-9-24(10-2)15-25(34)14-19(4)13-18(3)11-12-26(35)21(6)28(37)16-27(36)20(5)23(8)42-30(39)17-29(38)31-22(7)32(40)43-33(31)41/h9,15,18-21,23,26-27,29,35-36,38H,1,10-14,16-17H2,2-8H3/b24-15- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of protein phosphatase (PP)1 (IC50 values are 1.6 and 62 nM for PP1 and PP2 respectively). Reduces PDGF-induced vascular smooth muscle cell and mesangial cell proliferation without affecting fibronectin secretion and cellular kinase activation in vivo. Immunosuppressive agent; inhibits proliferation and induces apoptosis in activated T cells. |

Tautomycetin Dilution Calculator

Tautomycetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6481 mL | 8.2406 mL | 16.4813 mL | 32.9625 mL | 41.2031 mL |
| 5 mM | 0.3296 mL | 1.6481 mL | 3.2963 mL | 6.5925 mL | 8.2406 mL |
| 10 mM | 0.1648 mL | 0.8241 mL | 1.6481 mL | 3.2963 mL | 4.1203 mL |
| 50 mM | 0.033 mL | 0.1648 mL | 0.3296 mL | 0.6593 mL | 0.8241 mL |
| 100 mM | 0.0165 mL | 0.0824 mL | 0.1648 mL | 0.3296 mL | 0.412 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Schizanthine M
Catalog No.:BCN1939
CAS No.:119736-78-6
- Schizanthine G
Catalog No.:BCN1938
CAS No.:119736-74-2
- SB 206553 hydrochloride
Catalog No.:BCC7143
CAS No.:1197334-04-5
- SDZ 205-557 hydrochloride
Catalog No.:BCC7246
CAS No.:1197334-02-3
- Sazetidine A dihydrochloride
Catalog No.:BCC7468
CAS No.:1197329-42-2
- TGR5 Receptor Agonist
Catalog No.:BCC4195
CAS No.:1197300-24-5
- Baohuoside VII
Catalog No.:BCN2889
CAS No.:119730-89-1
- Fupenzic acid
Catalog No.:BCN6085
CAS No.:119725-20-1
- 2-Epitormentic acid
Catalog No.:BCN6084
CAS No.:119725-19-8
- UNC 0224
Catalog No.:BCC2430
CAS No.:1197196-48-7
- Sutchuenmedin A
Catalog No.:BCN6854
CAS No.:1197194-31-2
- PF-05212384 (PKI-587)
Catalog No.:BCC4987
CAS No.:1197160-78-3
- Baohuoside VI
Catalog No.:BCC8129
CAS No.:119760-73-5
- 29-Norlanosta-8,24-diene-1alpha,2alpha,3beta-triol
Catalog No.:BCN7984
CAS No.:119765-92-3
- 3-Furfuryl 2-pyrrolecarboxylate
Catalog No.:BCN6086
CAS No.:119767-00-9
- AP26113
Catalog No.:BCC1069
CAS No.:1197958-12-5
- Mirin
Catalog No.:BCC5986
CAS No.:1198097-97-0
- Sodium Channel inhibitor 1
Catalog No.:BCC1959
CAS No.:1198117-23-5
- Cerdulatinib (PRT062070)
Catalog No.:BCC8068
CAS No.:1198300-79-6
- Coptisine sulfate
Catalog No.:BCN2286
CAS No.:1198398-71-8
- Phyperunolide E
Catalog No.:BCN7292
CAS No.:1198400-52-0
- Esomeprazole magnesium salt
Catalog No.:BCC5430
CAS No.:1198768-91-0
- Posaconazole hydrate
Catalog No.:BCC4234
CAS No.:1198769-38-8
- CGRP 8-37 (human)
Catalog No.:BCC5724
CAS No.:119911-68-1
Thioesterase domain swapping of a linear polyketide tautomycetin with a macrocyclic polyketide pikromycin in Streptomyces sp. CK4412.[Pubmed:27277081]
J Ind Microbiol Biotechnol. 2016 Aug;43(8):1189-93.
Tautomycetin (TMC) is a linear polyketide metabolite produced by Streptomyces sp. CK4412 that has been reported to possess multiple biological functions including T cell-specific immunosuppressive and anticancer activities that occur through a mechanism of differential inhibition of protein phosphatases such as PP1, PP2A, and SHP2. We previously reported the characterization of the entire TMC biosynthetic gene cluster constituted by multifunctional type I polyketide synthase (PKS) assembly and suggested that the linear form of TMC could be generated via free acid chain termination by a narrow TMC thioesterase (TE) pocket. The modular nature of the assembly presents a unique opportunity to alter or interchange the native biosynthetic domains to produce targeted variants of TMC. Herein, we report swapping of the TMC TE domain sequence with the exact counterpart of the macrocyclic polyketide pikromycin (PIK) TE. PIK TE-swapped Streptomyces sp. CK4412 mutant produced not only TMC, but also a cyclized form of TMC, implying that the bioengineering based in vivo custom construct can be exploited to produce engineered macrolactones with new structural functionality.
TmcN is involved in ATP regulation of tautomycetin biosynthesis in Streptomyces griseochromogenes.[Pubmed:27444385]
Biochem Biophys Res Commun. 2016 Sep 9;478(1):221-226.
The regulatory mechanism of Tautomycetin (TMC) biosynthesis remains largely unknown, although it has been of great interest to the pharmaceutical industry. Our previous study showed that intracellular adenosine triphosphate (inATP) level is negatively correlated with secondary metabolite biosynthesis in various Streptomyces spp. In this study, by exogenous treatment of ATP, we also found a negative correlation between TMC biosynthesis and inATP level in Streptomyces griseochromogenes (S. griseochromogenes). However, the underlying mechanism remains unclear. TmcN, a pathway-specific transcriptional regulator of TMC biosynthetic genes, was previously revealed as a large ATP-binding LuxR (LAL) family protein. The predicted amino acid sequence of TmcN shows highly conserved Walker A and B binding motifs, which suggest an ATPase function of TmcN. We therefore hypothesized that the ATPase domain of TmcN may play a role in sensing endogenous pool of ATP, and is thus involved in the ATP regulation of TMC biosynthesis. To test the hypothesis, we first explored the key residue that affects the ATPase activity of TmcN by amino acid sequence alignment and structural simulation. After that, we disrupted tmcN gene in S. griseochromogenes, and the tmcN or site-direct-mutated tmcN were re-introduced to get the complementary and ATPase domain disrupted strains. The transcription level of tmcN, TMC yield, and inATP, as well as the effect of ATP on TMC production of different mutants were evaluated. Deletion of tmcN or site-direct mutation of ATPase domain of TmcN in S. griseochromogenes significantly reduced the TMC production, and it was not affected by exogenous ATP treatment. In addition, a relatively high level of inATP was detected in tmcN deletion and site-direct mutation strains. Our results here suggested that TmcN, especially its ATPase domain, is involved in consuming of endogenous ATP pool and thus plays pivotal role in connecting the primary and secondary metabolite in S. griseochromogenes.
Biosynthesis, regulation, and engineering of a linear polyketide tautomycetin: a novel immunosuppressant in Streptomyces sp. CK4412.[Pubmed:27734184]
J Ind Microbiol Biotechnol. 2017 May;44(4-5):555-561.
Tautomycetin (TMC) is a natural product with a linear structure that includes an ester bond connecting a dialkylmaleic moiety to a type I polyketide chain. Although TMC was originally identified as an antifungal antibiotic in the late 1980s, follow-up studies revealed its novel immunosuppressant activity. Specifically, TMC exhibited a mechanistically unique immunosuppressant activity about 100 times higher than that of cyclosporine A, a widely used immunosuppressant drug. Interestingly, a structurally close relative, tautomycin (TTM), was reported to not possess TMC-like immunosuppressant activity, suggesting that a distinctive polyketide moiety of TMC plays a critical role in immunosuppressant activity. Cloning and engineering of a TMC polyketide biosynthetic gene cluster generated several derivatives showing different biological activities. TMC was also found to be biosynthesized as a linear structure without forming a lactone ring, unlike the most polyketide-based compounds, implying the presence of a unique polyketide thioesterase in the cluster. Although TMC biosynthesis was limited due to its tight regulation by two pathway-specific regulatory genes located in the cluster, its production was significantly stimulated through homologous and heterologous expression of its entire biosynthetic gene cluster using a Streptomyces artificial chromosome vector system. In this mini-review, we summarize recent advances in the biosynthesis, regulation, and pathway engineering of a linear polyketide, TMC, in Streptomyces sp. CK4412.
Effects of tautomycetin on proliferation and fibronectin secretion in vascular smooth muscle cells and glomerular mesangial cells.[Pubmed:15919517]
Transplant Proc. 2005 May;37(4):1959-61.
Tautomycetin (TMC), a newly developed immunosuppressive agent, induces T-lymphocyte apoptosis through the inhibition of tyrosine kinase and protein phosphatase 1. We examined the effects of TMC on platelet-derived growth factor (PDGF)-induced proliferation and extracellular matrix synthesis in cultured vascular smooth muscle cells (VSMCs) and mesangial cells (MCs) of Sprague-Dawley rats, and investigated the molecular mechanisms involved. Different concentrations of TMC were administered 1 hour before the addition of 10 ng/mL PDGF into the growth-arrested and synchronized cells. Cell proliferation was assessed by methylthiazoletetrazolium (MTT) assay, fibronectin secretion, and the activation of Akt, ERK, and p38 MAPK by Western blot analysis. PDGF increased cell proliferation, fibronectin secretion, and the activation of Akt, ERK, and p38 MAPK in both VSMCs and MCs. In both cultured cells, TMC at >1 mug/mL significantly reduced basal MTT. TMC at 100 ng/mL significantly decreased the PDGF-induced VSMC and MC proliferation. However, fibronectin secretion and the activation of Akt, ERK, and p38 MAPK were not affected by this nontoxic concentration of TMC. The present data demonstrate that low-dose TMC reduced PDGF-induced VSMC and MC proliferation without affecting the fibronectin secretion and cellular kinase activation.
Immunosuppressive effects of tautomycetin in vivo and in vitro via T cell-specific apoptosis induction.[Pubmed:12149481]
Proc Natl Acad Sci U S A. 2002 Aug 6;99(16):10617-22.
Tautomycetin (TMC) was identified as an immunosuppressor of activated T cells. Inhibition of T cell proliferation with TMC was observed at concentrations 100-fold lower than those needed to achieve maximal inhibition with cyclosporin A (CsA). TMC specifically blocked tyrosine phosphorylation of intracellular signal mediators downstream of Src tyrosine kinases in a T cell-specific manner, leading to apoptosis due to cleavage of Bcl-2, caspase-9, caspase-3, and poly(ADP-ribose) polymerase, but not caspase-1. In TMC-treated rats that received a heterotopic cardiac allograft, the graft survived more than 160 days, comparable to graft survival in allografted rats treated with CsA. Thus, TMC, whose mechanism of action is different from that of CsA or FK506, can be used as a potent T cell-specific immunosuppressor.
Tautomycetin is a novel and specific inhibitor of serine/threonine protein phosphatase type 1, PP1.[Pubmed:11554729]
Biochem Biophys Res Commun. 2001 Sep 21;287(2):328-31.
Here we isolated Tautomycetin, TC, and examined its phosphatase inhibitory activity. Recently we have reported that the left-hand moiety of tautomycin, TM, and the right one containing the spiroketal are essentially required for inhibition of protein phosphatase, PP, and induction of apoptosis, respectively. TC is structurally almost identical to TM except that TC is lacking the spiroketal, which has the potential apoptosis-inducing activity. TC specifically inhibited PP1 activity, IC50 values for purified PP1 and PP2A enzymes being 1.6 and 62 nM, respectively, whereas the IC50 values of TM were 0.21 and 0.94 nM, respectively. These results demonstrate that TC is the most specific PP1 inhibitor out of over 40 species of natural phosphatase inhibitors reported, strongly suggesting that TC is a novel powerful tool to elucidate the physiological roles of PP1 in various biological events.


