UNC 0224G9a histone methyltransferase inhibitor CAS# 1197196-48-7 |
- SRT3109
Catalog No.:BCC1965
CAS No.:1204707-71-0
- Plerixafor 8HCl (AMD3100 8HCl)
Catalog No.:BCC4447
CAS No.:155148-31-5
- AMD 3465 hexahydrobromide
Catalog No.:BCC5182
CAS No.:185991-07-5
- Reparixin
Catalog No.:BCC1885
CAS No.:266359-83-5
- AMD-070
Catalog No.:BCC1357
CAS No.:558447-26-0
- SCH 546738
Catalog No.:BCC4110
CAS No.:906805-42-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

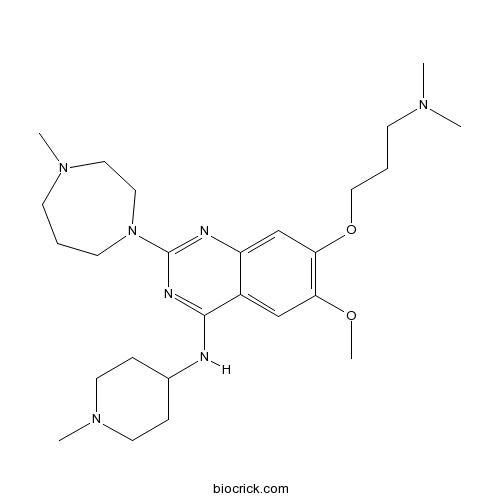
| Cas No. | 1197196-48-7 | SDF | Download SDF |
| PubChem ID | 44251522 | Appearance | Powder |
| Formula | C26H43N7O2 | M.Wt | 485.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. HCl and to 100 mM in DMSO | ||
| Chemical Name | 7-[3-(dimethylamino)propoxy]-6-methoxy-2-(4-methyl-1,4-diazepan-1-yl)-N-(1-methylpiperidin-4-yl)quinazolin-4-amine | ||
| SMILES | CN1CCCN(CC1)C2=NC3=CC(=C(C=C3C(=N2)NC4CCN(CC4)C)OC)OCCCN(C)C | ||
| Standard InChIKey | XIVUGRBSBIXXJE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H43N7O2/c1-30(2)10-7-17-35-24-19-22-21(18-23(24)34-5)25(27-20-8-13-32(4)14-9-20)29-26(28-22)33-12-6-11-31(3)15-16-33/h18-20H,6-17H2,1-5H3,(H,27,28,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent G9a and GLP inhibitor (IC50 values are 15 and 20 nM in a ThioGlo assay, respectively). Exhibits >1000-fold selectivity for G9a over SET7, SET8 and SET9. |

UNC 0224 Dilution Calculator

UNC 0224 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.059 mL | 10.2951 mL | 20.5901 mL | 41.1802 mL | 51.4753 mL |
| 5 mM | 0.4118 mL | 2.059 mL | 4.118 mL | 8.236 mL | 10.2951 mL |
| 10 mM | 0.2059 mL | 1.0295 mL | 2.059 mL | 4.118 mL | 5.1475 mL |
| 50 mM | 0.0412 mL | 0.2059 mL | 0.4118 mL | 0.8236 mL | 1.0295 mL |
| 100 mM | 0.0206 mL | 0.103 mL | 0.2059 mL | 0.4118 mL | 0.5148 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Potent inhibitor of G9a histone lysine methyltransferase (IC50 = 15 nM).
- Sutchuenmedin A
Catalog No.:BCN6854
CAS No.:1197194-31-2
- PF-05212384 (PKI-587)
Catalog No.:BCC4987
CAS No.:1197160-78-3
- 5-Methylfurmethiodide
Catalog No.:BCC6707
CAS No.:1197-60-0
- 4-Aminophenylacetic acid
Catalog No.:BCC8687
CAS No.:1197-55-3
- Tranexamic acid
Catalog No.:BCN2710
CAS No.:1197-18-8
- 3',4'-Dihydroxyacetophenone
Catalog No.:BCN4775
CAS No.:1197-09-7
- Amadacycline methanesulfonate
Catalog No.:BCC1356
CAS No.:1196800-40-4
- Yucalexin P-17
Catalog No.:BCN6595
CAS No.:119642-82-9
- Moguisteine
Catalog No.:BCC4925
CAS No.:119637-67-1
- Naloxone benzoylhydrazone
Catalog No.:BCC5757
CAS No.:119630-94-3
- Arecaidine but-2-ynyl ester tosylate
Catalog No.:BCC6627
CAS No.:119630-77-2
- Sulfo-NHS-Biotin
Catalog No.:BCC3576
CAS No.:119616-38-5
- 2-Epitormentic acid
Catalog No.:BCN6084
CAS No.:119725-19-8
- Fupenzic acid
Catalog No.:BCN6085
CAS No.:119725-20-1
- Baohuoside VII
Catalog No.:BCN2889
CAS No.:119730-89-1
- TGR5 Receptor Agonist
Catalog No.:BCC4195
CAS No.:1197300-24-5
- Sazetidine A dihydrochloride
Catalog No.:BCC7468
CAS No.:1197329-42-2
- SDZ 205-557 hydrochloride
Catalog No.:BCC7246
CAS No.:1197334-02-3
- SB 206553 hydrochloride
Catalog No.:BCC7143
CAS No.:1197334-04-5
- Schizanthine G
Catalog No.:BCN1938
CAS No.:119736-74-2
- Schizanthine M
Catalog No.:BCN1939
CAS No.:119736-78-6
- Tautomycetin
Catalog No.:BCC7320
CAS No.:119757-73-2
- Baohuoside VI
Catalog No.:BCC8129
CAS No.:119760-73-5
- 29-Norlanosta-8,24-diene-1alpha,2alpha,3beta-triol
Catalog No.:BCN7984
CAS No.:119765-92-3
The associations between environmental quality and preterm birth in the United States, 2000-2005: a cross-sectional analysis.[Pubmed:26051702]
Environ Health. 2015 Jun 9;14:50.
BACKGROUND: Many environmental factors have been independently associated with preterm birth (PTB). However, exposure is not isolated to a single environmental factor, but rather to many positive and negative factors that co-occur. The environmental quality index (EQI), a measure of cumulative environmental exposure across all US counties from 2000-2005, was used to investigate associations between ambient environment and PTB. METHODS: With 2000-2005 birth data from the National Center for Health Statistics for the United States (n = 24,483,348), we estimated the association between increasing quintiles of the EQI and county-level and individual-level PTB; we also considered environmental domain-specific (air, water, land, sociodemographic and built environment) and urban-rural stratifications. RESULTS: Effect estimates for the relationship between environmental quality and PTB varied by domain and by urban-rural strata but were consistent across county- and individual-level analyses. The county-level prevalence difference (PD (95% confidence interval) for the non-stratified EQI comparing the highest quintile (poorest environmental quality) to the lowest quintile (best environmental quality) was -0.0166 (-0.0198, -0.0134). The air and sociodemographic domains had the strongest associations with PTB; PDs were 0.0196 (0.0162, 0.0229) and -0.0262 (-0.0300, -0.0224) for the air and sociodemographic domain indices, respectively. Within the most urban strata, the PD for the sociodemographic domain index was 0.0256 (0.0205, 0.0307). Odds ratios (OR) for the individual-level analysis were congruent with PDs. CONCLUSION: We observed both strong positive and negative associations between measures of broad environmental quality and preterm birth. Associations differed by rural-urban stratum and by the five environmental domains. Our study demonstrates the use of a large scale composite environment exposure metric with preterm birth, an important indicator of population health and shows potential for future research.
Discovery of a 2,4-diamino-7-aminoalkoxyquinazoline as a potent and selective inhibitor of histone lysine methyltransferase G9a.[Pubmed:19891491]
J Med Chem. 2009 Dec 24;52(24):7950-3.
SAR exploration of the 2,4-diamino-6,7-dimethoxyquinazoline template led to the discovery of 8 (UNC0224) as a potent and selective G9a inhibitor. A high resolution X-ray crystal structure of the G9a-8 complex, the first cocrystal structure of G9a with a small molecule inhibitor, was obtained. The cocrystal structure validated our binding hypothesis and will enable structure-based design of novel inhibitors. 8 is a useful tool for investigating the biology of G9a and its roles in chromatin remodeling.


