3',4'-DihydroxyacetophenoneCAS# 1197-09-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1197-09-7 | SDF | Download SDF |
| PubChem ID | 14530 | Appearance | Powder |
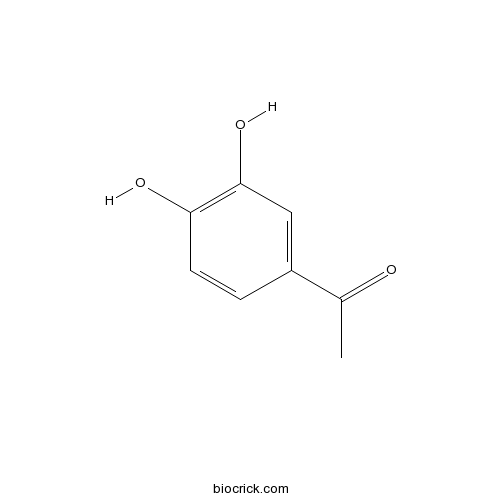
| Formula | C8H8O3 | M.Wt | 152.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(3,4-dihydroxyphenyl)ethanone | ||
| SMILES | CC(=O)C1=CC(=C(C=C1)O)O | ||
| Standard InChIKey | UCQUAMAQHHEXGD-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 3',4'-Dihydroxyacetophenone has antimicrobial activity. |
| Targets | Antifection |

3',4'-Dihydroxyacetophenone Dilution Calculator

3',4'-Dihydroxyacetophenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.5703 mL | 32.8515 mL | 65.703 mL | 131.406 mL | 164.2576 mL |
| 5 mM | 1.3141 mL | 6.5703 mL | 13.1406 mL | 26.2812 mL | 32.8515 mL |
| 10 mM | 0.657 mL | 3.2852 mL | 6.5703 mL | 13.1406 mL | 16.4258 mL |
| 50 mM | 0.1314 mL | 0.657 mL | 1.3141 mL | 2.6281 mL | 3.2852 mL |
| 100 mM | 0.0657 mL | 0.3285 mL | 0.657 mL | 1.3141 mL | 1.6426 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Amadacycline methanesulfonate
Catalog No.:BCC1356
CAS No.:1196800-40-4
- Yucalexin P-17
Catalog No.:BCN6595
CAS No.:119642-82-9
- Moguisteine
Catalog No.:BCC4925
CAS No.:119637-67-1
- Naloxone benzoylhydrazone
Catalog No.:BCC5757
CAS No.:119630-94-3
- Arecaidine but-2-ynyl ester tosylate
Catalog No.:BCC6627
CAS No.:119630-77-2
- Sulfo-NHS-Biotin
Catalog No.:BCC3576
CAS No.:119616-38-5
- Olprinone Hydrochloride
Catalog No.:BCC1821
CAS No.:119615-63-3
- PF-3845
Catalog No.:BCC2326
CAS No.:1196109-52-0
- GSK2190915 sodium salt
Catalog No.:BCC5588
CAS No.:1196070-26-4
- 2-Hydroxyquinoxaline
Catalog No.:BCC8577
CAS No.:1196-57-2
- 7-Ethyl-10-Hydroxy-Camptothecin
Catalog No.:BCN8386
CAS No.:119577-28-5
- Dabrafenib Mesylate (GSK-2118436)
Catalog No.:BCC1513
CAS No.:1195768-06-9
- Tranexamic acid
Catalog No.:BCN2710
CAS No.:1197-18-8
- 4-Aminophenylacetic acid
Catalog No.:BCC8687
CAS No.:1197-55-3
- 5-Methylfurmethiodide
Catalog No.:BCC6707
CAS No.:1197-60-0
- PF-05212384 (PKI-587)
Catalog No.:BCC4987
CAS No.:1197160-78-3
- Sutchuenmedin A
Catalog No.:BCN6854
CAS No.:1197194-31-2
- UNC 0224
Catalog No.:BCC2430
CAS No.:1197196-48-7
- 2-Epitormentic acid
Catalog No.:BCN6084
CAS No.:119725-19-8
- Fupenzic acid
Catalog No.:BCN6085
CAS No.:119725-20-1
- Baohuoside VII
Catalog No.:BCN2889
CAS No.:119730-89-1
- TGR5 Receptor Agonist
Catalog No.:BCC4195
CAS No.:1197300-24-5
- Sazetidine A dihydrochloride
Catalog No.:BCC7468
CAS No.:1197329-42-2
- SDZ 205-557 hydrochloride
Catalog No.:BCC7246
CAS No.:1197334-02-3
Preparation of dual-responsive hybrid fluorescent nano probe based on graphene oxide and boronic acid/BODIPY-conjugated polymer for cell imaging.[Pubmed:27987660]
Mater Sci Eng C Mater Biol Appl. 2017 Feb 1;71:1064-1071.
Here, we report a pH- and thermo-responsive fluorescent nanomaterial of functionalized reduced graphene oxide (rGO) with cross-linked polymer produced via catechol-boronate diol binding mechanism. When conjugated with the hydrophobic dye boron dipyrromethane (BODIPY), this material can act as a dual-responsive nanoplatform for cells imaging. 2-Chloro-3',4'-dihydroxyacetophenone (CCDP)-quaternized-poly(dimethylaminoethyl methacrylate-co-N-isopropylacrylamide) [C-PDN] was cross-linked with BODIPY and 4-chlorophenyl boronic acid (BA)-quaternized-poly(ethylene glycol)-g-poly(dimethylaminoethyl methacrylate-co-N-isopropylacrylamide) [BB-PPDN]. The GO was then reduced by the catechol group in the cross-linked polymer to synthesize rGO nanoparticles, which able to stabilize the quenching mechanism. This nanoplatform exhibits intense fluorescence at acidic pH and low fluorescence at physiological pH. Confocal laser scanning microscopy (CLSM) images shows bright fluorescence at lysosomal pH and total quench at physiological pH. Therefore, we have successfully developed a promising sensitive bio-imaging probe for identifying cancer cells.
Successful stabilization of functionalized hybrid graphene for high-performance antimicrobial activity.[Pubmed:23602878]
Acta Biomater. 2013 Aug;9(8):7996-8003.
We have prepared an antimicrobial nanocomposite composed of reduced graphene oxide (rGO) using antimicrobial agents and catechol derivative conjugated to polyethylene glycol-grafted poly(dimethylaminoethyl methacrylate) (PEG-g-PDMA). Graphene oxide (GO) has been simultaneously reduced by 2-chloro-3',4'-dihydroxyacetophenone (CCDP) in Tris buffer at pH 8.5 following catechol chemistry. Both CCDP and antimicrobial agent 1-bromododecane (C12) were quaternized to PEG-g-PDMA (CCDP-C12)-q-(PEG-g-PDMA). This synthesized polymer functionalized rGO as an antimicrobial nanocomposite, rGO/(CCDP-C12)-q-(PEG-g-PDMA). To increase antimicrobial activity, silver nanoparticles (Ag NPs) were deposited onto the high surface area of rGO/(CCDP-C12)-q-(PEG-g-PDMA). The prepared antimicrobial nanocomposite shows significant stability in aqueous media due to the hydrophilic behaviour of PEG. X-ray photoelectron spectroscopy investigation clearly shows the quaternization of C-12 and deposition of Ag NPs onto rGO surfaces. Ag NP-deposited rGO/(CCDP-C12)-q-(PEG-g-PDMA) shows better antimicrobial activity both against Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli bacteria at lower concentration compared to without applying Ag NPs. Investigation of the cytotoxicity demonstrates outstanding non-toxic properties of both the prepared nanocomposite as well as the synthesized polymer.
Maple syrup phytochemicals include lignans, coumarins, a stilbene, and other previously unreported antioxidant phenolic compounds.[Pubmed:21033720]
J Agric Food Chem. 2010 Nov 24;58(22):11673-9.
Twenty-three phenolic compounds were isolated from a butanol extract of Canadian maple syrup (MS-BuOH) using chromatographic methods. The compounds were identified from their nuclear magnetic resonance and mass spectral data as 7 lignans [lyoniresinol (1), secoisolariciresinol (2), dehydroconiferyl alcohol (3), 5'-methoxy-dehydroconiferyl alcohol (4), erythro-guaiacylglycerol-beta-O-4'-coniferyl alcohol (5), erythro-guaiacylglycerol-beta-O-4'-dihydroconiferyl alcohol (6), and [3-[4-[(6-deoxy-alpha-l-mannopyranosyl)oxy]-3-methoxyphenyl]methyl]-5-(3,4-dimeth oxyphenyl)dihydro-3-hydroxy-4-(hydroxymethyl)-2(3H)-furanone (7)], 2 coumarins [scopoletin (8) and fraxetin (9)], a stilbene [(E)-3,3'-dimethoxy-4,4'-dihydroxystilbene (10)], and 13 phenolic derivatives [2-hydroxy-3',4'-dihydroxyacetophenone (11), 1-(2,3,4-trihydroxy-5-methylphenyl)ethanone (12), 2,4,5-trihydroxyacetophenone (13), catechaldehyde (14), vanillin (15), syringaldehyde (16), gallic acid (17), trimethyl gallic acid methyl ester (18), syringic acid (19), syringenin (20), (E)-coniferol (21), C-veratroylglycol (22), and catechol (23)]. The antioxidant activities of MS-BuOH (IC50>1000 mug/mL), pure compounds, vitamin C (IC50=58 muM), and a synthetic commercial antioxidant, butylated hydroxytoluene (IC50=2651 muM), were evaluated in the diphenylpicrylhydrazyl (DPPH) radical scavenging assay. Among the isolates, the phenolic derivatives and coumarins showed superior antioxidant activity (IC50<100 muM) compared to the lignans and stilbene (IC50>100 muM). Also, this is the first report of 16 of these 23 phenolics, that is, compounds 1, 2, 4-14, 18, 20, and 22, in maple syrup.
Synergistic effects of thiols and amines on antiradical efficiency of protocatechuic acid.[Pubmed:15612812]
J Agric Food Chem. 2004 Dec 29;52(26):8163-8.
DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity of protocatechuic acid and its structural analogues (methyl protocatechuate, 3',4'-dihydroxyacetophenone, 3,4-dihydroxybenzaldehyde, and 3,4-dihydroxybenzonitrile) were examined in aprotic and protic solvents. In aprotic acetonitrile, all test compounds scavenged two radicals. In protic methanol, however, these compounds rapidly scavenged five radicals except for protocatechuic acid, which consumed only two radicals. The result indicated that higher radical scavenging activity in methanol than in acetonitrile was due to a nucleophilic addition of the methanol molecule on the oxidized quinones, which led to a regeneration of catechol structures. To investigate the importance of the nucleophilic addition on the quinones for the high radical scavenging activity, DPPH radical scavenging activity of protocatechuic acid and its analogues was examined in the presence of a variety of nucleophiles. The addition of a strong nucleophile such as a cysteine derivative significantly increased the radical scavenging equivalence. Furthermore, thiol adducts at C-2 and C-2,5 of protocatechuic acid and its analogues were isolated from the reaction mixtures. These results strongly suggest that the quinone of protocatechuic acid and its analogues undergo a nucleophilic attack at C-2 to yield 2-substituted-3,4-diols. Then, a regenerated catechol moiety of adducts scavenge two additional radicals by reoxidation into quinones, which undergo the second nucleophilic attack at the C-5. This mechanism demonstrates a possibility of synergistic effects of various nucleophiles on the radical scavenging ability of plant polyphenols containing a 3,4-dihydroxy substructure like protocatechuic acid and its analogues.
4-alkyl-o-quinone/2-hydroxy-p-quinone methide isomerase from the larval hemolymph of Sarcophaga bullata. I. Purification and characterization of enzyme-catalyzed reaction.[Pubmed:2211605]
J Biol Chem. 1990 Oct 5;265(28):16992-9.
An enzyme which catalyzes the conversion of certain 4-alkyl-o-benzoquinones to 2-hydroxy-p-quinone methides has been purified to apparent homogeneity from the hemolymph of Sarcophaga bullata by employing conventional protein purification techniques. The purified enzyme migrated with an approximate molecular weight of 98,000 on gel filtration chromatography. On sodium dodecyl sulfate-polyacrylamide gel electrophoresis, it migrated as a single band with a molecular weight of 46,000, indicating that it is made up of two identical subunits. It exhibited a pH optimum of 6.0 and readily converted chemically synthesized as well as enzymatically generated quinones derived from N-acetyldopamine, N-beta-alanyldopamine, and 3,4-dihydroxyphenethyl alcohol to highly unstable 2-hydroxy-p-quinone methides. The quinone methides thus formed were rapidly and nonenzymatically hydrated to form side chain hydroxylated o-diphenols as the stable product. In support of this proposition, when the enzyme reaction with N-acetyldopamine quinone was conducted in the presence of 10% methanol, racemic beta-methoxy-N-acetyldopamine was recovered as an additional product. The quinones of N-acetylnorepinephrine, N-beta-alanylnorepinephrine, and 3,4-dihydroxyphenylglycol were also attacked by the isomerase, resulting in the formation of N-acetylarterenone, N-beta-alanylarterenone and 2-hydroxy-3',4'-dihydroxyacetophenone, respectively as the stable products. The isomerase converted the dihydrocaffeiyl methyl amide quinone to its quinone methide analog which rapidly tautomerized to yield caffeiyl methyl amide. The importance of quinone isomerase in insect immunity and sclerotization of insect cuticle is discussed.
Biosynthesis of dehydro-N-acetyldopamine by a soluble enzyme preparation from the larval cuticle of Sarcophaga bullata involves intermediary formation of N-acetyldopamine quinone and N-acetyldopamine quinone methide.[Pubmed:2134025]
Arch Insect Biochem Physiol. 1990;15(4):237-54.
The enzymes involved in the side chain hydroxylation and side chain desaturation of the sclerotizing precursor N-acetyldopamine (NADA) were obtained in the soluble form from the larval cuticle of Sarcophaga bullata and the mechanism of the reaction was investigated. Phenylthiourea, a well-known inhibitor of phenoloxidases, drastically inhibited both the reactions, indicating the requirement of a phenoloxidase component. N-acetylcysteine, a powerful quinone trap, trapped the transiently formed NADA quinone and prevented the production of both N-acetylnorepinephrine and dehydro NADA. Exogenously added NADA quinone was readily converted by these enzyme preparations to N-acetylnorepinephrine and dehydro NADA. 4-Alkyl-o-quinone:2-hydroxy-p-quinone methide isomerase obtained from the cuticular preparations converted chemically synthesized NADA quinone to its quinone methide. The quinone methide formed reacted rapidly and nonenzymatically with water to form N-acetylnorepinephrine as the stable product. Similarly 4-(2-hydroxyethyl)-o-benzoquinone was converted to 3,4-dihydroxyphenyl glycol. When the NADA quinone-quinone isomerase reaction was performed in buffer containing 10% methanol, beta-methoxy NADA was obtained as an additional product. Furthermore, the quinones of N-acetylnorepinephrine and 3,4-dihydroxyphenyl glycol were converted to N-acetylarterenone and 2-hydroxy-3',4'-dihydroxyacetophenone, respectively, by the enzyme. Comparison of nonenzymatic versus enzymatic transformation of NADA to N-acetylnorepinephrine revealed that the enzymatic reaction is at least 100 times faster than the nonenzymatic rate. Resolution of the NADA desaturase system on Benzamidine Sepharose and Sephacryl S-200 columns yielded the above-mentioned quinone isomerase and NADA quinone methide:dehydro NADA isomerase. The latter, on reconstitution with mushroom tyrosinase and hemolymph quinone isomerase, catalyzed the biosynthesis of dehydro NADA from NADA with the intermediary formation of NADA quinone and NADA quinone methide. The results are interpreted in terms of the quinone methide model elaborated by our group [Sugumaran: Adv. Insect Physiol. 21:179-231, 1988; Sugumaran et al.: Arch. Insect Biochem. Physiol. 11:109, 1989] and it is concluded that the two enzyme beta-sclerotization model [Andersen: Insect Biochem. 19:59-67, 375-382, 1989] is inadequate to account for various observations made on insect cuticle.


