Dabrafenib Mesylate (GSK-2118436)Inhibitor of BRAF(V600) mutants CAS# 1195768-06-9 |
- CEP-32496
Catalog No.:BCC1079
CAS No.:1188910-76-0
- Dabrafenib (GSK2118436)
Catalog No.:BCC4393
CAS No.:1195765-45-7
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- BRAF inhibitor
Catalog No.:BCC1436
CAS No.:918505-61-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1195768-06-9 | SDF | Download SDF |
| PubChem ID | 44516822 | Appearance | Powder |
| Formula | C24H24F3N5O5S3 | M.Wt | 615.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GSK2118436 Mesylate; GSK 2118436B | ||
| Solubility | DMSO : ≥ 36 mg/mL (58.47 mM) *"≥" means soluble, but saturation unknown. | ||
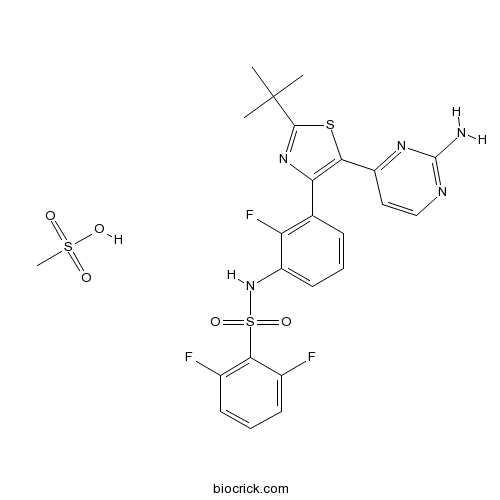
| Chemical Name | N-[3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide;methanesulfonic acid | ||
| SMILES | CC(C)(C)C1=NC(=C(S1)C2=NC(=NC=C2)N)C3=C(C(=CC=C3)NS(=O)(=O)C4=C(C=CC=C4F)F)F.CS(=O)(=O)O | ||
| Standard InChIKey | YKGMKSIHIVVYKY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H20F3N5O2S2.CH4O3S/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25;1-5(2,3)4/h4-11,31H,1-3H3,(H2,27,28,29);1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Targets | Raf | ||||
| IC50 | 3.2/0.8/5.0 nM (B-Raf/B-RafV600E/ c-Raf) |
| Cell experiment [1]: | |
| Cell lines | M257 wild-type BRAF, LCP BRAFV600R and WM266 BRAFV600D melanoma cell lines. |
| Preparation method | Soluble in DMSO. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 3-100 nM; 72 h |
| Applications | Dabrafenib remarkably inhibits cell proliferation and phosphorylated ERK in both melanoma cell lines carrying a mutated BRAF. |
| Human experiment [2]: | |
| Patients | Female CD1 nu/nu mice xenografted BRAFV600E (A375P) human tumor. |
| Dosage form | 30 mg/kg; 14 days; dosed orally once daily. |
| Preparation method | 0.5% hydroxypropylmethylcellulose, 0.2% Tween 80 in pH 8.0 distilled water; 0.2 mL per 20 g of bodyweight. |
| Application | Dabrafenib is orally bioavailable, reduces pERK and inhibits tumor growth. Dabrafenib reduces pERK and Ki67 by 89% and 28% respectively and increases p27 by 54%. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1]. Gentilcore G, Madonna G, Mozzillo N, et al. Effect of dabrafenib on melanoma cell lines harbouring the BRAF(V600D/R) mutations. BMC Cancer, 2013, 13: 17. [2]. King AJ, Arnone MR, Bleam MR, et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS One, 2013, 8(7): e67583. | |

Dabrafenib Mesylate (GSK-2118436) Dilution Calculator

Dabrafenib Mesylate (GSK-2118436) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6242 mL | 8.1212 mL | 16.2425 mL | 32.4849 mL | 40.6062 mL |
| 5 mM | 0.3248 mL | 1.6242 mL | 3.2485 mL | 6.497 mL | 8.1212 mL |
| 10 mM | 0.1624 mL | 0.8121 mL | 1.6242 mL | 3.2485 mL | 4.0606 mL |
| 50 mM | 0.0325 mL | 0.1624 mL | 0.3248 mL | 0.6497 mL | 0.8121 mL |
| 100 mM | 0.0162 mL | 0.0812 mL | 0.1624 mL | 0.3248 mL | 0.4061 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
The pharmacokinetic parameters of dabrafenib, an inhibitor of human BRAF, CRAF and mutant BRAF kinases, have determined in four patients with BRAF mutation-positive solid tumors, in which the least squares mean absolute bioavailability, median T(max) after oral administration, the geometric mean terminal half-life, the geometric mean clearance and volume of distribution after IV administration were 94.5%, 2.0 hours, 4.8 hours, 12.0 L/h and 45.5 L respectively.
Abstract
Dabrafenib has been assessed for IC and EC patterns of response and progression in the treatment of patients with active melanoma brain metastases.
Abstract
The population pharmacokinetics of dabrfenib, a BRAF inhibitor, were characterized, in which steady state was achieved in 14 days following a 150 mg BID dose with total clearance, induction half-life and pre-dose concentration of 34.3 L/h, 67 hours and 46.6 ng/mL respectively. Capsule shell, sex and weight did affect dabrafenib exposure; while age, renal and hepatic impairment didn’t.
Abstract
Dabrafenib exhibits efficacy against BRAF V600E-mutated melanoma, non-V600E BRAF-mutated disease and brain metastases, where 50% response rate and 6 months progression-free survival were observed in dabrafenib-treated melanoma patients with BRAF V600E mutations. The combination of dabrafenib and trametinib showed synergistic effects to improve both the progression-free survival and overall survival of melanoma patients.
Abstract
Clinical development and characteristics of dabrafenib were summarized and compared to vemurafenib.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK2118436 is a selective BRAF V600E inhibitor. BRAF encodes a proto-oncogene B-Raf also known as serine/threonine protein kinase B-Raf. It is critical in regulating the MAPK/ERK signaling pathway. BRAF mutations frequently occur in many human cancers. [1, 2] BRAF V600E mutant is constitutively active, allowing MAPK/ERK activation independent of upstream cues. [3]
GSK2118436 binds to Raf family kinases and inhibits their activity. It is highly selective against B-Raf V600E with IC50 of 0.8 nM, compared to wild type B-Raf and c-Raf with IC50s of 3.2 nM and 5.0 nM, respectively. [4]
GSK2118436 treatment shows selective inhibition of MAPK/ERK activation, proliferation, transformation and tumorigenicity. FDA approved GSK2118436 as a single agent treatment for advanced melanoma with BRAF V600E mutation on May 30, 2013.
GSK2118436 can be taken orally.
References:
[1]Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, Ohtsuru A, Saenko VA, Kanematsu T, Yamashita S. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J. Clin. Endocrinol. Metab. 2003; 88 (9): 4393–7.
[2]Tan YH, Liu Y, Eu KW, Ang PW, Li WQ, Salto-Tellez M, Iacopetta B, Soong R. Detection of BRAF V600E mutation by pyrosequencing. Pathology 2008; 40 (3): 295–8.
[3]Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 417: 949-954.
[4]Ma XH, Piao SF, Dey S, McAfee Q, Karakousis G, Villanueva J, Hart LS, Levi S, Hu J, Zhang G, Lazova R, Klump V, Pawelek JM, Xu X, Xu W, Schuchter LM, Davies MA, Herlyn M, Winkler J, Koumenis C, Amaravadi RK. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J Clin Invest. 2014; 124(3): 1406-17.
- Dabrafenib (GSK2118436)
Catalog No.:BCC4393
CAS No.:1195765-45-7
- 11-Hydroxygelsenicine
Catalog No.:BCN4761
CAS No.:1195760-68-9
- N,N-Dimethylsphingosine
Catalog No.:BCC7959
CAS No.:119567-63-4
- Othonnine
Catalog No.:BCN2061
CAS No.:119565-25-2
- Ceanothic acid acetate
Catalog No.:BCN6083
CAS No.:119533-63-0
- Ethyllucidone
Catalog No.:BCN6082
CAS No.:1195233-59-0
- Meropenem trihydrate
Catalog No.:BCC4226
CAS No.:119478-56-7
- Fruquintinib(HMPL-013)
Catalog No.:BCC6415
CAS No.:1194506-26-7
- Eliprodil
Catalog No.:BCC7280
CAS No.:119431-25-3
- Loureirin B
Catalog No.:BCN5021
CAS No.:119425-90-0
- Loureirin A
Catalog No.:BCN3671
CAS No.:119425-89-7
- Galanin (1-30) (human)
Catalog No.:BCC6961
CAS No.:119418-04-1
- 7-Ethyl-10-Hydroxy-Camptothecin
Catalog No.:BCN8386
CAS No.:119577-28-5
- 2-Hydroxyquinoxaline
Catalog No.:BCC8577
CAS No.:1196-57-2
- GSK2190915 sodium salt
Catalog No.:BCC5588
CAS No.:1196070-26-4
- PF-3845
Catalog No.:BCC2326
CAS No.:1196109-52-0
- Olprinone Hydrochloride
Catalog No.:BCC1821
CAS No.:119615-63-3
- Sulfo-NHS-Biotin
Catalog No.:BCC3576
CAS No.:119616-38-5
- Arecaidine but-2-ynyl ester tosylate
Catalog No.:BCC6627
CAS No.:119630-77-2
- Naloxone benzoylhydrazone
Catalog No.:BCC5757
CAS No.:119630-94-3
- Moguisteine
Catalog No.:BCC4925
CAS No.:119637-67-1
- Yucalexin P-17
Catalog No.:BCN6595
CAS No.:119642-82-9
- Amadacycline methanesulfonate
Catalog No.:BCC1356
CAS No.:1196800-40-4
- 3',4'-Dihydroxyacetophenone
Catalog No.:BCN4775
CAS No.:1197-09-7
Cutaneous Toxic Effects of BRAF Inhibitors Alone and in Combination With MEK Inhibitors for Metastatic Melanoma.[Pubmed:26200476]
JAMA Dermatol. 2015 Oct;151(10):1103-9.
IMPORTANCE: The cutaneous adverse effects of the BRAF inhibitors vemurafenib and dabrafenib mesylate in the treatment of metastatic melanoma have been well reported. The addition of a MEK inhibitor to a BRAF inhibitor improves the blockade of the mitogen-activated protein kinase pathway. The combination of dabrafenib with the MEK inhibitor trametinib dimethyl sulfoxide (CombiDT therapy) increases response rate and survival compared with a BRAF inhibitor alone. Clinical trials have suggested that CombiDT therapy induces fewer cutaneous toxic effects than a single-agent BRAF inhibitor. To our knowledge, a direct comparison has not been performed before. OBJECTIVE: To compare the cutaneous toxic effects of BRAF inhibitor monotherapy and CombiDT therapy in a large cohort of patients. DESIGN, SETTING, AND PARTICIPANTS: We performed a retrospective cohort study from September 1, 2009, through November 30, 2013. The study population included 185 Australian patients with unresectable stages IIIC and IV melanoma referred from Crown Princess Mary Cancer Care Centre who underwent review at the Department of Dermatology, Westmead Hospital. Of these, 119 patients received dabrafenib; 36, vemurafenib; and 30, CombiDT therapy. Data analysis were performed in December 2013. MAIN OUTCOMES AND MEASURES: Multiple cutaneous adverse effects between BRAF inhibitor monotherapy and CombiDT therapy were identified and compared in a cohort of patients who underwent the same dermatologic assessment. RESULTS: The most common cutaneous adverse effects seen in patients receiving the single-agent BRAF inhibitor dabrafenib or vemurafenib included Grover disease (51 patients [42.9%] and 14 [38.9%], respectively [P = .67]), plantar hyperkeratosis (47 [39.5%] and 14 [38.9%], respectively [P = .95]), verrucal keratosis (79 [66.4%] and 26 [72.2%], respectively [P = .51]), and cutaneous squamous cell carcinoma (31 [26.1%] and 13 [36.1%], respectively [P = .54]). Photosensitivity was more common with vemurafenib (14 patients [38.9%]) compared with dabrafenib (1 [0.8%]; P < .001). Compared with dabrafenib, CombiDT therapy showed a higher frequency of folliculitis (12 patients [40.0%] vs. 8 [6.7%]; P < .001) and a significant decrease of cutaneous squamous cell carcinoma (0 vs. 31 [26.1%]; P < .001), verrucal keratosis (0 vs. 79 [66.4%]; P < .001), and Grover disease (0 vs. 51 [42.9%]; P < .001). CONCLUSIONS AND RELEVANCE: This study confirms that the prevalence of cutaneous toxic effects differs among vemurafenib, dabrafenib, and CombiDT therapies. Cutaneous squamous cell carcinoma is the most concerning cutaneous toxic effect related to BRAF inhibitor monotherapy that did not appear with CombiDT therapy. Although CombiDT therapy has an improved profile of cutaneous toxic effects, continuous dermatologic assessments should be provided for all patients when receiving these treatments.
Pharmaceutical approval update.[Pubmed:24391391]
P T. 2013 Nov;38(11):705-7.
Vortioxetine (Brintellix) for major depressive disorder; ustekinumab (stelara) for psoriatic arthritis; and dabrafenib mesylate (Tafinlar) for metastatic melanoma.
The GIST of targeted therapy for malignant melanoma.[Pubmed:24531699]
Ann Surg Oncol. 2014 Jun;21(6):2059-67.
The high response rates to the tyrosine kinase inhibitor imatinib in KIT-mutated gastrointestinal stromal tumors (GIST) has led to a paradigm shift in cancer treatment. In a parallel fashion, the field of melanoma is shifting with the utilization of targeted therapy to treat BRAF-mutated melanoma. We reviewed published literature in PubMed on GIST and melanoma, with a focus on both past and current clinical trials. The data presented centers on imatinib, vemurafenib, and most recently dabrafenib, targeting KIT and BRAF mutations and their outcomes in GIST and melanoma. The BRAF(V600E) melanoma mutation, like the KIT exon 11 mutation in GIST, has the highest response to therapy. High response rates with inhibition of KIT in GIST have not been recapitulated in KIT-mutated melanoma. Median time to resistance to targeted agents occurs in ~7 months with BRAF inhibitors and 2 years for imatinib in GIST. In GIST, the development of secondary mutations leads to resistance; however, there have been no similar gatekeeper mutations found in melanoma. Although surgery remains an important component of the treatment of early GIST and melanoma, surgeons will need to continue to define the thresholds and timing for operation in the setting of metastatic disease with improved targeted therapies. Combination treatment strategies may result in more successful clinical outcomes in the management of melanoma in the future.
Low MITF/AXL ratio predicts early resistance to multiple targeted drugs in melanoma.[Pubmed:25502142]
Nat Commun. 2014 Dec 15;5:5712.
Increased expression of the Microphthalmia-associated transcription factor (MITF) contributes to melanoma progression and resistance to BRAF pathway inhibition. Here we show that the lack of MITF is associated with more severe resistance to a range of inhibitors, while its presence is required for robust drug responses. Both in primary and acquired resistance, MITF levels inversely correlate with the expression of several activated receptor tyrosine kinases, most frequently AXL. The MITF-low/AXL-high/drug-resistance phenotype is common among mutant BRAF and NRAS melanoma cell lines. The dichotomous behaviour of MITF in drug response is corroborated in vemurafenib-resistant biopsies, including MITF-high and -low clones in a relapsed patient. Furthermore, drug cocktails containing AXL inhibitor enhance melanoma cell elimination by BRAF or ERK inhibition. Our results demonstrate that a low MITF/AXL ratio predicts early resistance to multiple targeted drugs, and warrant clinical validation of AXL inhibitors to combat resistance of BRAF and NRAS mutant MITF-low melanomas.
[Melanoma: from molecular studies to the treatment breakthrough].[Pubmed:24341237]
Arkh Patol. 2013 Sep-Oct;75(5):63-72.
Melanoma holds a leading position in the mortality from skin tumors. Standard treatment of metastatic melanoma allows tumor remission to be achieved only in a small subset of patients. Studies on melanoma molecular pathogenesis led to the identification of several causative genetic events and, consequently, to the development of novel targeted drugs. More than a half of melanomas contain amine acid substitutions in serine-threonine kinase BRAF. Clinical trials involving specific BRAF inhibitors--vemurafenib and dabrafenib--demonstrated high efficacy of these agents towards BRAF-mutated melanoma. MEK inhibitors may show activity against both BRAF--and NRAS-driven tumors. Mucosal and acral melanomas frequently contain mutation in KIT receptor and can be successfully treated by imatinib. There are novel therapeutic monoclonal antibodies targeted against immunosuppressive molecules CTLA4, PD-1 and PD-L1. In some instances these drugs allow to obtain exceptionally prolonged responses. Whole genome sequencing led to the identification of new melanoma genes, e.g. GRIN2A, TRRAP, PREX2, RAC1, STK19, PPP6C, etc. Molecular testing, especially BRAF mutation analysis, has become a mandatory part of melanoma diagnosis. Nevertheless, despite the revolution in melanoma treatment, the prevention of excessive ultraviolet exposure, cancer awareness and early diagnosis remain the main tools for the management of this disease.


