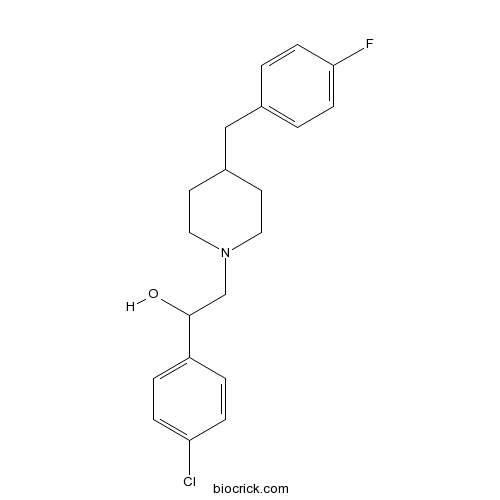
EliprodilCAS# 119431-25-3 |
- XL-888
Catalog No.:BCC2339
CAS No.:1149705-71-4
- MKT 077
Catalog No.:BCC6241
CAS No.:147366-41-4
- Alvespimycin
Catalog No.:BCC1346
CAS No.:467214-20-6
- NVP-BEP800
Catalog No.:BCC2129
CAS No.:847559-80-2
- BIIB021
Catalog No.:BCC2124
CAS No.:848695-25-0
- PF-04929113 (SNX-5422)
Catalog No.:BCC2130
CAS No.:908115-27-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 119431-25-3 | SDF | Download SDF |
| PubChem ID | 60703 | Appearance | Powder |
| Formula | C20H23ClFNO | M.Wt | 347.86 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SL-820715 | ||
| Solubility | DMSO : 14.29 mg/mL (41.08 mM; Need ultrasonic) | ||
| Chemical Name | 1-(4-chlorophenyl)-2-[4-[(4-fluorophenyl)methyl]piperidin-1-yl]ethanol | ||
| SMILES | C1CN(CCC1CC2=CC=C(C=C2)F)CC(C3=CC=C(C=C3)Cl)O | ||
| Standard InChIKey | GGUSQTSTQSHJAH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H23ClFNO/c21-18-5-3-17(4-6-18)20(24)14-23-11-9-16(10-12-23)13-15-1-7-19(22)8-2-15/h1-8,16,20,24H,9-14H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-competitive NMDA receptor antagonist that acts at the polyamine modulatory site. Selective for NR2B- over NR2A- and NR2C-containing receptors (IC50 values are 1, > 100 and > 100 μM respectively). Also σ1 ligand (Ki = 0.013 μM). Antagonizes neuronal voltage-gated Ca2+ channels and selectively inhibits the rapid component of the delayed rectifier K+ current (IKr). Neuroprotective. |

Eliprodil Dilution Calculator

Eliprodil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8747 mL | 14.3736 mL | 28.7472 mL | 57.4944 mL | 71.868 mL |
| 5 mM | 0.5749 mL | 2.8747 mL | 5.7494 mL | 11.4989 mL | 14.3736 mL |
| 10 mM | 0.2875 mL | 1.4374 mL | 2.8747 mL | 5.7494 mL | 7.1868 mL |
| 50 mM | 0.0575 mL | 0.2875 mL | 0.5749 mL | 1.1499 mL | 1.4374 mL |
| 100 mM | 0.0287 mL | 0.1437 mL | 0.2875 mL | 0.5749 mL | 0.7187 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Eliprodil(SL-820715) is a non-competitive NR2B-NMDA receptor antagonist(IC50=1 uM), less potent for NR2A- and NR2C-containing receptors(IC50> 100 uM). IC50 value: Target: NR2B-NMDA antagonist Human N-type Ca2+ channel currents were inhibited by ifenprodil and eliprodil with IC50 values of 50 microM and 10 microM respectively whereas P-type Ca2+ channel currents were inhibited reversibly by ifenprodil and eliprodil with approximate IC50 values of 60 microM and 9 microM respectively. eliprodil (1 microm) produced a moderate reverse rate-dependent prolongation of the action potential duration (7.4+/-1.5, 8.9+/-2.1 and 9.9+/-1.8% at cycle lengths of 300, 1000 and 5000 ms, respectively; n=9).
References:
[1]. Bath CP, et al. The effects of ifenprodil and eliprodil on voltage-dependent Ca2+ channels and in gerbil global cerebral ischaemia. Eur J Pharmacol. 1996 Mar 28;299(1-3):103-12.
[2]. Lengyel C, et al. Effect of a neuroprotective drug, eliprodil on cardiac repolarisation: importance of the decreased repolarisation reserve in the development of proarrhythmic risk. Br J Pharmacol. 2004 Sep;143(1):152-8.
- Loureirin B
Catalog No.:BCN5021
CAS No.:119425-90-0
- Loureirin A
Catalog No.:BCN3671
CAS No.:119425-89-7
- Galanin (1-30) (human)
Catalog No.:BCC6961
CAS No.:119418-04-1
- Licoricesaponin E2
Catalog No.:BCN7894
CAS No.:119417-96-8
- Topotecan hydrochloride
Catalog No.:BCN2604
CAS No.:119413-54-6
- LY2811376
Catalog No.:BCC2102
CAS No.:1194044-20-6
- Przewalskin
Catalog No.:BCN6080
CAS No.:119400-87-2
- Salviolone
Catalog No.:BCN3141
CAS No.:119400-86-1
- 2,5-Dihydroxybenzaldehyde
Catalog No.:BCN6081
CAS No.:1194-98-5
- mAChR-IN-1
Catalog No.:BCC5512
CAS No.:119391-56-9
- 2-Deacetoxytaxinine J
Catalog No.:BCN7291
CAS No.:119347-14-7
- CRANAD 2
Catalog No.:BCC6293
CAS No.:1193447-34-5
- Fruquintinib(HMPL-013)
Catalog No.:BCC6415
CAS No.:1194506-26-7
- Meropenem trihydrate
Catalog No.:BCC4226
CAS No.:119478-56-7
- Ethyllucidone
Catalog No.:BCN6082
CAS No.:1195233-59-0
- Ceanothic acid acetate
Catalog No.:BCN6083
CAS No.:119533-63-0
- Othonnine
Catalog No.:BCN2061
CAS No.:119565-25-2
- N,N-Dimethylsphingosine
Catalog No.:BCC7959
CAS No.:119567-63-4
- 11-Hydroxygelsenicine
Catalog No.:BCN4761
CAS No.:1195760-68-9
- Dabrafenib (GSK2118436)
Catalog No.:BCC4393
CAS No.:1195765-45-7
- Dabrafenib Mesylate (GSK-2118436)
Catalog No.:BCC1513
CAS No.:1195768-06-9
- 7-Ethyl-10-Hydroxy-Camptothecin
Catalog No.:BCN8386
CAS No.:119577-28-5
- 2-Hydroxyquinoxaline
Catalog No.:BCC8577
CAS No.:1196-57-2
- GSK2190915 sodium salt
Catalog No.:BCC5588
CAS No.:1196070-26-4
Synthesis and resolution of racemic eliprodil and evaluation of the enantiomers of eliprodil as NMDA receptor antagonists.[Pubmed:10890168]
Bioorg Med Chem Lett. 2000 Jun 19;10(12):1377-80.
A short synthesis of the NMDA receptor antagonist (rac)-Eliprodil (9) and its resolution into the enantiomers by chiral HPLC is described. The enantiomers (R)-9 and (S)-9 were found to exhibit markedly different affinities for NR2B subunit containing NMDA receptors.
Administration of eliprodil during ethanol withdrawal in the neonatal rat attenuates ethanol-induced learning deficits.[Pubmed:15064913]
Psychopharmacology (Berl). 2004 Sep;175(2):189-95.
RATIONALE: Prenatal exposure to alcohol can disrupt brain development, leading to a variety of behavioral alterations, including learning deficits. We have postulated that some central nervous system damage may be due to N-methyl-D-aspartate (NMDA) receptor-mediated excitotoxicity that occurs during ethanol withdrawal. Consistent with this hypothesis, we previously demonstrated that administration of MK-801, an NMDA receptor antagonist, during ethanol withdrawal attenuates ethanol-related learning deficits using an animal model of fetal alcohol effects. However, MK-801 binds to the phencyclidine site, which affects all NMDA receptor subtypes and can cause adverse side effects and toxicity. Eliprodil is a more selective NMDA receptor antagonist that acts at the polyamine modulatory site of NMDA receptors. OBJECTIVES: The purpose of this study was to determine if administration of Eliprodil during ethanol withdrawal would reduce the severity of learning deficits associated with developmental alcohol exposure. METHODS: Male rat pups were randomly assigned to ethanol-exposed or control treatments. On postnatal day (PD) 6, during a period of brain development similar to that of the mid-third trimester in humans, subjects were exposed to 6.0 g/kg ethanol or isocaloric maltose solutions via oral gavage. Twenty-four hours after the end of the ethanol treatment, during ethanol withdrawal, all subjects received an intraperitoneal injection of one of three doses of Eliprodil (5, 10, or 25 mg/kg) or vehicle. On PD 40, all subjects were tested on a serial spatial discrimination reversal learning task. RESULTS: Ethanol-exposed subjects treated with vehicle committed a significantly greater number of errors compared to controls. Administration of Eliprodil during ethanol withdrawal significantly decreased the number of errors in the ethanol-exposed groups, but had no significant effect on the performance of controls. CONCLUSION: These data support the hypothesis that NMDA receptor-mediated excitotoxicity during ethanol withdrawal contributes to fetal alcohol effects.
Effect of a neuroprotective drug, eliprodil on cardiac repolarisation: importance of the decreased repolarisation reserve in the development of proarrhythmic risk.[Pubmed:15302678]
Br J Pharmacol. 2004 Sep;143(1):152-8.
1. The aim of this study was to analyse the effects of Eliprodil, a noncardiac drug with neuroprotective properties, on the cardiac repolarisation under in vitro circumstances, under normal conditions and after the attenuation of the 'repolarisation reserve' by blocking the inward rectifier potassium current (I(K1)) current with BaCl(2). 2. In canine right ventricular papillary muscle by applying the conventional microelectrode technique, under normal conditions, Eliprodil (1 microm) produced a moderate reverse rate-dependent prolongation of the action potential duration (7.4+/-1.5, 8.9+/-2.1 and 9.9+/-1.8% at cycle lengths of 300, 1000 and 5000 ms, respectively; n=9). 3. This effect was augmented in preparations where I(K1) was previously blocked by BaCl(2) (10 microm). BaCl(2) alone lengthened APD in a reverse frequency-dependent manner (7.0+/-1.3, 14.2+/-1.6 and 28.1+/-2.1% at cycle lengths of 300, 1000 and 5000 ms, respectively; n=8). When Eliprodil (1 microm) was administered to these preparations, the drug induced a marked further lengthening relative to the APD values measured after the administration of BaCl(2) (12.5+/-1.0, 17.6+/-1.5 and 20.5+/-0.9% at cycle lengths of 300, 1000 and 5000 ms, respectively; n=8). 4. In the normal Langendorff-perfused rabbit heart, Eliprodil (1 microm) produced a significant QT(c) prolongation at 1 Hz stimulation frequency (12.7+/-1.8%, n=9). After the attenuation of the 'repolarisation reserve' by the I(K1) blocker BaCl(2) (10 microm), the Eliprodil-evoked QT(c) prolongation was greatly enhanced (28.5+/-7.9%, n=6). In two out of six Langendorff preparations, this QT(c) lengthening degenerated into torsade de pointes ventricular tachycardia. 5. Eliprodil significantly decreased the amplitude of rapid component of the delayed rectifier potassium current (I(Kr)), but slow component (I(Ks)), transient outward current (I(to)) and I(K1) were not considerably affected by the drug when measured in dog ventricular myocytes by applying the whole-cell configuration of the patch-clamp technique. 6. The results indicate that Eliprodil, under normal conditions, moderately lengthens cardiac repolarisation by inhibition of I(Kr). However, after the attenuation of the normal 'repolarisation reserve', this drug can induce marked QT interval prolongation, which may result in proarrhythmic action.
Neuroprotective effects of eliprodil in retinal excitotoxicity and ischemia.[Pubmed:10235551]
Invest Ophthalmol Vis Sci. 1999 May;40(6):1177-82.
PURPOSE: To evaluate whether Eliprodil (SL82.0715), a NR2B-selective N-methyl-D-aspartate (NMDA) antagonist, is protective of retina subjected to an excitotoxic or ischemic insult. METHODS: To evaluate protection against retinal excitotoxicity, Eliprodil was administered intraperitoneally before and after the injection of NMDA (5 microl, 20 nmol) into the vitreous of rats. Integrity of the retina was assessed by counting cells in the retinal ganglion cell layer (GCL) and measuring choline acetyltransferase (ChAT) activity. In a subsequent experiment, total retinal ischemia, as measured by a cessation of electroretinographic (ERG) activity, was induced in anesthetized rabbits by elevating intraocular pressure above systolic blood pressure for 65 minutes. After ischemia, recovery of ERG activity was assessed at 24 and 48 hours in animals treated with vehicle or Eliprodil (1.0-10.0 mg/kg). RESULTS: Intravitreal NMDA injection resulted in a dose-related decrease in cells of the GCL and in ChAT activity. Eliprodil administered intraperitoneally at 10 mg/kg completely prevented the loss of ChAT and the loss of cells in the GCL. Twenty-four hours after retinal ischemia, A and B waves of vehicle-treated animals were suppressed by 60% to 70%. Eliprodil administered intraperitoneally at 10 mg/kg ameliorated the A- and B-wave depression throughout the 48-hour experiment. CONCLUSIONS: Eliprodil is neuroprotective of retinae subjected to either an excitotoxic or ischemic challenge and may be useful for treating a variety of retinal and optic nerve head disorders.
Antagonism of N-methyl-D-aspartate receptors by sigma site ligands: potency, subtype-selectivity and mechanisms of inhibition.[Pubmed:9223571]
J Pharmacol Exp Ther. 1997 Jul;282(1):326-38.
Recent studies propose that sigma site ligands antagonize N-methyl-D-aspartate (NMDA) receptors by either direct, or indirect mechanisms of inhibition. To investigate this question further we used electrical recordings to assay actions of seventeen structurally diverse sigma site ligands on three diheteromeric subunit combinations of cloned rat NMDA receptors expressed in Xenopus oocytes: NR1a coexpressed with either NR2A, 2B or 2C. The sigma site ligands had a wide range of potency for antagonizing NMDA receptor currents. Steady-state IC50 values ranged between approximately 0.1 to >100 microM. In all cases inhibition was non-competitive with respect to glycine and glutamate. Five structurally related sigma ligands [Eliprodil, haloperidol, ifenprodil, 4-phenyl-1-(4-phenylbutyl)-piperidine and trifluperidol] were strongly selective for NR1a/2B receptors. The other drugs were weakly selective or nonselective inhibitors. There was no correlation between sigma site affinity and potency of NMDA receptor antagonism for any subunit combination. Inhibition of NR1a/2B receptors by the selective antagonists was independent of voltage whereas inhibition by the weakly selective antagonists was voltage dependent. Potency of 10 sigma ligands was cross-checked on NMDA currents in cultured rat cortical neurons. There was close correspondence between the two assay systems. Our results argue that antagonism of NMDA receptor currents by the sigma ligands tested is due to direct effects on the receptor channel complex as opposed to indirect effects mediated by sigma receptors. Inhibition occurs via sites in the NMDA receptor channel pore, or via allosteric modulatory sites associated with the NR2B subunit.
The effects of ifenprodil and eliprodil on voltage-dependent Ca2+ channels and in gerbil global cerebral ischaemia.[Pubmed:8901012]
Eur J Pharmacol. 1996 Mar 28;299(1-3):103-12.
Ifenprodil and Eliprodil are both non-competitive NMDA receptor antagonists which have been shown to inhibit neuronal Ca2+ channel currents. We have examined the effects of these agents on two defined subtypes of voltage-dependent Ca2+ channels and in the gerbil model of global cerebral ischaemia. Recombinantly expressed human alpha 1B-1 alpha 2b beta 1-3 Ca2+ subunits in HEK293 cells, which results in an omega-conotoxin-sensitive neuronal N-type voltage-dependent Ca2+ channel and omega-Aga IVA sensitive Ca2+ channels (P-type) in acutely isolated cerebellar Purkinje neurones were reversibly inhibited by ifenprodil and Eliprodil. Human N-type Ca2+ channel currents were inhibited by ifenprodil and Eliprodil with IC50 values of 50 microM and 10 microM respectively whereas P-type Ca2+ channel currents were inhibited reversibly by ifenprodil and Eliprodil with approximate IC50 values of 60 microM and 9 microM respectively. Maximum current block observed for both channel subtypes was approximately 80% for both ifenprodil and Eliprodil. For neuroprotection studies, animals were subjected to 5 min bilateral carotid artery occlusion with or without administration of either ifenprodil or Eliprodil (5, 10 or 20 mg/kg i.p.) immediately after surgery followed by two further doses (2.5, 5 or 10 mg/kg, respectively) at 3 and 6 h post-occlusion. Both compounds provided significant protective effects against ischaemia-induced neurodegeneration in the CA1 region of the hippocampus. These results indicate that both ifenprodil and Eliprodil protect against ischaemia-induced neurodegeneration when administered post-occlusion and that they also block N and P-type voltage-dependent Ca2+ channels.


