2,5-DihydroxybenzaldehydeCAS# 1194-98-5 |
Quality Control & MSDS
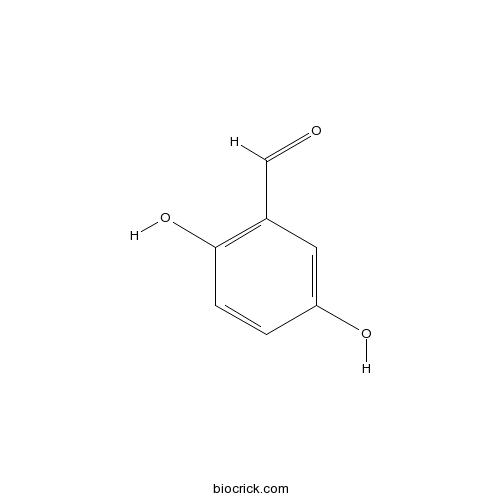
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1194-98-5 | SDF | Download SDF |
| PubChem ID | 70949 | Appearance | Powder |
| Formula | C7H6O3 | M.Wt | 138.1 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,5-dihydroxybenzaldehyde | ||
| SMILES | C1=CC(=C(C=C1O)C=O)O | ||
| Standard InChIKey | CLFRCXCBWIQVRN-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 2,5-Dihydroxybenzaldehyde has antioxidant activity. |
| Targets | Antifection |
| In vitro | Effect of structure on the interactions between five natural antimicrobial compounds and phospholipids of bacterial cell membrane on model monolayers.[Pubmed: 24914896]Molecules. 2014 Jun 6;19(6):7497-515.Monolayers composed of bacterial phospholipids were used as model membranes to study interactions of the naturally occurring phenolic compounds 2,5-Dihydroxybenzaldehyde and 2-hydroxy-5-methoxybenzaldehyde, and the plant essential oil compounds carvacrol, cinnamaldehyde, and geraniol, previously found to be active against both Gram-positive and Gram-negative pathogenic microorganisms. Diffusion-free mediator based miniature biofuel cell anode fabricated on a carbon-MEMS electrode.[Pubmed: 22946444 ]Langmuir. 2012 Oct 2;28(39):14055-64.We report on the functionalization of a micropatterned carbon electrode fabricated using the carbon-MEMS process for its use as a miniature diffusion-free glucose oxidase anode. |
| Structure Identification | Int J Biol Macromol. 2012 Dec;51(5):1159-66.Synthesis and characterization of novel nano-chitosan Schiff base and use of lead (II) sensor.[Pubmed: 22982811]A new kind of nano-chitosan Schiff base ligand (CHNS) with particle size of 34 nm was formed by the reaction between the 2-amino groups of glucosamine residue of nano-chitosan and a 2,5-Dihydroxybenzaldehyde. Comptes Rendus Chimie, 2016,20(4): 365-9.Spectroscopic determination of the dissociation constants of 2,4- and 2,5-dihydroxybenzaldehydes and relationships to their antioxidant activities[Reference: WebLink]
|

2,5-Dihydroxybenzaldehyde Dilution Calculator

2,5-Dihydroxybenzaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.2411 mL | 36.2056 mL | 72.4113 mL | 144.8226 mL | 181.0282 mL |
| 5 mM | 1.4482 mL | 7.2411 mL | 14.4823 mL | 28.9645 mL | 36.2056 mL |
| 10 mM | 0.7241 mL | 3.6206 mL | 7.2411 mL | 14.4823 mL | 18.1028 mL |
| 50 mM | 0.1448 mL | 0.7241 mL | 1.4482 mL | 2.8965 mL | 3.6206 mL |
| 100 mM | 0.0724 mL | 0.3621 mL | 0.7241 mL | 1.4482 mL | 1.8103 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- mAChR-IN-1
Catalog No.:BCC5512
CAS No.:119391-56-9
- 2-Deacetoxytaxinine J
Catalog No.:BCN7291
CAS No.:119347-14-7
- CRANAD 2
Catalog No.:BCC6293
CAS No.:1193447-34-5
- 2-Hydroxydiplopterol
Catalog No.:BCN7290
CAS No.:1193250-54-2
- Olean-12-ene-3,24-diol
Catalog No.:BCN6079
CAS No.:119318-15-9
- 2-Benzyl-2-(dimethylamino)-4'-morpholinobutyrophenone
Catalog No.:BCC8563
CAS No.:119313-12-1
- Pinobanksin 5-methyl ether
Catalog No.:BCN7775
CAS No.:119309-36-3
- Atalantoflavone
Catalog No.:BCN4857
CAS No.:119309-02-3
- Rocuronium Bromide
Catalog No.:BCC1068
CAS No.:119302-91-9
- PE 154
Catalog No.:BCC7858
CAS No.:1192750-33-6
- Scutellarin methylester
Catalog No.:BCN2828
CAS No.:119262-68-9
- Avibactam
Catalog No.:BCC1384
CAS No.:1192500-31-4
- Salviolone
Catalog No.:BCN3141
CAS No.:119400-86-1
- Przewalskin
Catalog No.:BCN6080
CAS No.:119400-87-2
- LY2811376
Catalog No.:BCC2102
CAS No.:1194044-20-6
- Topotecan hydrochloride
Catalog No.:BCN2604
CAS No.:119413-54-6
- Licoricesaponin E2
Catalog No.:BCN7894
CAS No.:119417-96-8
- Galanin (1-30) (human)
Catalog No.:BCC6961
CAS No.:119418-04-1
- Loureirin A
Catalog No.:BCN3671
CAS No.:119425-89-7
- Loureirin B
Catalog No.:BCN5021
CAS No.:119425-90-0
- Eliprodil
Catalog No.:BCC7280
CAS No.:119431-25-3
- Fruquintinib(HMPL-013)
Catalog No.:BCC6415
CAS No.:1194506-26-7
- Meropenem trihydrate
Catalog No.:BCC4226
CAS No.:119478-56-7
- Ethyllucidone
Catalog No.:BCN6082
CAS No.:1195233-59-0
Effect of structure on the interactions between five natural antimicrobial compounds and phospholipids of bacterial cell membrane on model monolayers.[Pubmed:24914896]
Molecules. 2014 Jun 6;19(6):7497-515.
Monolayers composed of bacterial phospholipids were used as model membranes to study interactions of the naturally occurring phenolic compounds 2,5-Dihydroxybenzaldehyde and 2-hydroxy-5-methoxybenzaldehyde, and the plant essential oil compounds carvacrol, cinnamaldehyde, and geraniol, previously found to be active against both Gram-positive and Gram-negative pathogenic microorganisms. The lipid monolayers consist of 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (DPPE), 1,2-dihexa- decanoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (DPPG), and 1,1',2,2'-tetratetradecanoyl cardiolipin (cardiolipin). Surface pressure-area (pi-A) and surface potential-area (Deltapsi-A) isotherms were measured to monitor changes in the thermodynamic and physical properties of the lipid monolayers. Results of the study indicated that the five compounds modified the three lipid monolayer structures by integrating into the monolayer, forming aggregates of antimicrobial -lipid complexes, reducing the packing effectiveness of the lipids, increasing the membrane fluidity, and altering the total dipole moment in the monolayer membrane model. The interactions of the five antimicrobial compounds with bacterial phospholipids depended on both the structure of the antimicrobials and the composition of the monolayers. The observed experimental results provide insight into the mechanism of the molecular interactions between naturally-occurring antimicrobial compounds and phospholipids of the bacterial cell membrane that govern activities.
Diffusion-free mediator based miniature biofuel cell anode fabricated on a carbon-MEMS electrode.[Pubmed:22946444]
Langmuir. 2012 Oct 2;28(39):14055-64.
We report on the functionalization of a micropatterned carbon electrode fabricated using the carbon-MEMS process for its use as a miniature diffusion-free glucose oxidase anode. Carbon-MEMS based electrodes offer precise manufacturing control on both the micro- and nanoscale and possess higher electron conductivity than redox hydrogels. However, the process involves pyrolysis in a reducing environment that renders the electrode surface less reactive and introduction of a high density of functional groups becomes challenging. Our functionalization strategy involves the electrochemical oxidation of amine linkers onto the electrode. This strategy works well with both aliphatic and aryl linkers and uses stable compounds. The anode is designed to operate through mediated electron transfer between 2,5-Dihydroxybenzaldehyde (DHB) based redox mediator and glucose oxidase enzyme. The electrode was first functionalized with ethylene diamine (EDA) to serve as a linker for the redox mediator. The redox mediator was then grafted through reductive amination, and attachment was confirmed through cyclic voltammetry. The enzyme immobilization was carried out through either adsorption or attachment, and their efficiency was compared. For enzyme attachment, the DHB attached electrode was functionalized again through electro-oxidation of aminobenzoic acid (ABA) linker. The ABA functionalization resulted in reduction of the DHB redox current, perhaps due to increased steric hindrance on the electrode surface, but the mediator function was preserved. Enzyme attachment was then carried out through a coupling reaction between the free carboxyl group on the ABA linker and the amine side chains on the enzyme. The enzyme incubation for both adsorption and attachment was done either through a dry spotting method or wet spotting method. The dry spotting method calls for the evaporation of enzyme droplet to form a thin film before sealing the electrode environment, to increase the effective concentration of the enzyme on the electrode surface during incubation. The electrodes were finally protected with a gelatin based hydrogel film. The anode half-cell was tested using cyclic voltammetry in deoxygenated phosphate buffer saline solution pH 7.4 to minimize oxygen interference and to simulate the pH environment of the body. The electrodes that yielded the highest anodic current were prepared by enzyme attachment method with dry spotting incubation. A polarization response was generated for this anodic half-cell and exhibits operation close to maximum efficiency that is limited by the mass transport of glucose to the electrode.
A bioanode based on MWCNT/protein-assisted co-immobilization of glucose oxidase and 2,5-dihydroxybenzaldehyde for glucose fuel cells.[Pubmed:20472420]
Biosens Bioelectron. 2010 Jul 15;25(11):2515-21.
This paper describes an easy-to-prepare, robust bioanode constructed on a polyester-supported screen-printed carbon paste electrode (SPCE) for glucose biofuel cells. To prepare the bioanode, carboxylated multi-walled carbon nanotubes (MWCNTs) were drop-coated on the SPCE first, and then a crosslinked matrix composed of glucose oxidase (GOx), 2,5-Dihydroxybenzaldehyde (DHB), bovine serum albumin (BSA) and glutaraldehyde was coated atop the MWCNTs. It was found that the MWCNTs assisted the immobilization of the crosslinked matrix, enhanced the electron-shuttling process, and showed electrocatalytic effect to gluconic acid, which allowed squeeze more electrons out of a glucose molecule. Inside the matrix, DHB mediators could couple to GOx and BSA via the Schiff base reaction, and GOx and BSA could crosslink to each other with glutaraldehyde. From cyclic voltammetry, it was estimated that 3.63 nmol cm(-2) of DHB was anchored on the bioanode, and no mediator leaching was observed. The bioanode also attained reproducible flow-injection analysis (FIA) signals for glucose sensing (RSD=4.99%) and retained 84% of the initial response after keeping in a buffer at 4 degrees C for a week. In addition, the bioanode obeyed the Michaelis-Menten kinetics. Finally, we demonstrated that a glucose biofuel cell assembled with an optimal bioanode and a laccase/ABTS cathode generated an electric power of 45 microW cm(-2) from 1M glucose at 37 degrees C.
Synthesis and characterization of novel nano-chitosan Schiff base and use of lead (II) sensor.[Pubmed:22982811]
Int J Biol Macromol. 2012 Dec;51(5):1159-66.
A new kind of nano-chitosan Schiff base ligand (CHNS) with particle size of 34 nm was formed by the reaction between the 2-amino groups of glucosamine residue of nano-chitosan and a 2,5-Dihydroxybenzaldehyde. The chemical structures of the nano-chitosan and nano-chitosan Schiff base were characterized by FT-IR spectra, particle sizer, zeta potential, and elemental analysis. A new, simple and effective chemically modified carbon paste electrode with CHNS was prepared and used as a lead (II) sensor. The prepared electrode was characterized using scanning electronic microscopy (SEM-EDX) and cyclic voltammetry (CV). The modified electrode showed only one oxidation peak in the anodic scan at -0.34 V (vs. Ag/AgCl) for the oxidation of lead (II). The dedection limit (LOD) was calculated as 1.36x10(-7) for a 10-min preconcentration time at pH 6.0.


