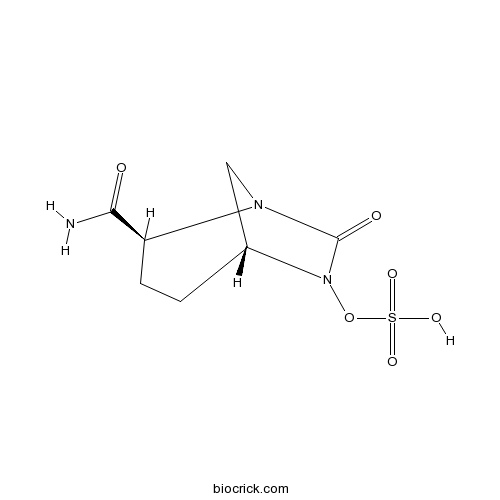
AvibactamBeta-lactamase inhibitor CAS# 1192500-31-4 |
- PD123319
Catalog No.:BCC5010
CAS No.:130663-39-7
- Irbesartan
Catalog No.:BCC2560
CAS No.:138402-11-6
- AVE 0991 sodium salt
Catalog No.:BCC4222
CAS No.:306288-04-0
- Perindopril
Catalog No.:BCC4223
CAS No.:82834-16-0
- Azilsartan medoxomil monopotassium
Catalog No.:BCC4089
CAS No.:863031-24-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1192500-31-4 | SDF | Download SDF |
| PubChem ID | 9835049 | Appearance | Powder |
| Formula | C7H11N3O6S | M.Wt | 265.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO | ||
| Chemical Name | [(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl] hydrogen sulfate | ||
| SMILES | C1CC(N2CC1N(C2=O)OS(=O)(=O)O)C(=O)N | ||
| Standard InChIKey | NDCUAPJVLWFHHB-UHNVWZDZSA-N | ||
| Standard InChI | InChI=1S/C7H11N3O6S/c8-6(11)5-2-1-4-3-9(5)7(12)10(4)16-17(13,14)15/h4-5H,1-3H2,(H2,8,11)(H,13,14,15)/t4-,5+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Avibactam free acid is a covalent, reversible β-lactamase inhibitor, inhibits β-lactamase TEM-1 and CTX-M-15 with IC50 of 8 nM and 5 nM, respectively.In Vitro:Avibactam (NXL104) is a molecule with little antibacterial activity, that inhibits class A and C β-lactamases. Avibactam inactivates most important β-lactamases except metallo types and Acinetobacter OXA carbapenemases[2].In Vivo:Avibactam sodium displays a slow return of activity with an off-rate of 0.045±0.022 min-1, which converts to a residence time half-life (tt1/2) of 16±8 min. The measured off-rate for Avibactam suggests that slow deacylation through hydrolysis or reversibility is occurring, and it is in contrast to previously reported extremely long t1/2 values of >1 or >7 d for Avibactam inhibition of TEM-1[1]. Avibactam is a new promising β-lactamase inhibitor, to overcome resistance caused by β-lactamases. Mice are infected with ca.106 CFU of Pseudomonas aeruginosa intramuscularly into the thigh or intranasally to cause pneumonia and are given 8 different (single) subcutaneous doses of Ceftazidime and Avibactam in various combined concentrations, ranging from 1 to 128 mg/kg of body weight in 2-fold increases. The mean estimated half-life in plasma of Ceftazidime in the terminal phase is 0.28 h (SD, 0.02 h), and that of Avibactam is 0.24 h (SD, 0.04 h). Volumes of distribution are 0.80 liters/kg (SD, 0.14 liters/kg) and 1.18 liters/kg (SD, 0.34 liters/kg), respectively[3]. References: | |||||

Avibactam Dilution Calculator

Avibactam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7702 mL | 18.8509 mL | 37.7017 mL | 75.4034 mL | 94.2543 mL |
| 5 mM | 0.754 mL | 3.7702 mL | 7.5403 mL | 15.0807 mL | 18.8509 mL |
| 10 mM | 0.377 mL | 1.8851 mL | 3.7702 mL | 7.5403 mL | 9.4254 mL |
| 50 mM | 0.0754 mL | 0.377 mL | 0.754 mL | 1.5081 mL | 1.8851 mL |
| 100 mM | 0.0377 mL | 0.1885 mL | 0.377 mL | 0.754 mL | 0.9425 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Avibactam is a novel investigational non-beta-lactam beta-lactamase inhibitor that is being developed for possible use in combination with ceftaroline in the U.S. Avibactam does not have any intrinsic antibacterial activity in its own right, but appears to be capable of inhibiting beta-lactamase enzymes that belong to molecular classes A and C.Avibactam is useful for Antibiotics.
- Gomisin S
Catalog No.:BCN3622
CAS No.:119239-49-5
- LX7101 HCL
Catalog No.:BCC6414
CAS No.:1192189-69-7
- PHT-427
Catalog No.:BCC2554
CAS No.:1191951-57-1
- 4-(1H-1,2,4-Triazol-1-ylmethyl)aniline
Catalog No.:BCC8645
CAS No.:119192-10-8
- CZC 54252 hydrochloride
Catalog No.:BCC6218
CAS No.:1191911-27-9
- CZC-25146
Catalog No.:BCC5371
CAS No.:1191911-26-8
- 10-Hydroxyscandine
Catalog No.:BCN6078
CAS No.:119188-47-5
- Coronarin B
Catalog No.:BCN6077
CAS No.:119188-38-4
- Coronarin D
Catalog No.:BCN6076
CAS No.:119188-37-3
- Coronarin A
Catalog No.:BCN6075
CAS No.:119188-33-9
- Glychionide A
Catalog No.:BCN3250
CAS No.:119152-50-0
- 3-Oxosapriparaquinone
Catalog No.:BCN3153
CAS No.:119139-56-9
- Scutellarin methylester
Catalog No.:BCN2828
CAS No.:119262-68-9
- PE 154
Catalog No.:BCC7858
CAS No.:1192750-33-6
- Rocuronium Bromide
Catalog No.:BCC1068
CAS No.:119302-91-9
- Atalantoflavone
Catalog No.:BCN4857
CAS No.:119309-02-3
- Pinobanksin 5-methyl ether
Catalog No.:BCN7775
CAS No.:119309-36-3
- 2-Benzyl-2-(dimethylamino)-4'-morpholinobutyrophenone
Catalog No.:BCC8563
CAS No.:119313-12-1
- Olean-12-ene-3,24-diol
Catalog No.:BCN6079
CAS No.:119318-15-9
- 2-Hydroxydiplopterol
Catalog No.:BCN7290
CAS No.:1193250-54-2
- CRANAD 2
Catalog No.:BCC6293
CAS No.:1193447-34-5
- 2-Deacetoxytaxinine J
Catalog No.:BCN7291
CAS No.:119347-14-7
- mAChR-IN-1
Catalog No.:BCC5512
CAS No.:119391-56-9
- 2,5-Dihydroxybenzaldehyde
Catalog No.:BCN6081
CAS No.:1194-98-5
Ceftazidime-avibactam: novel antimicrobial combination for the treatment of complicated urinary tract infections.[Pubmed:28338347]
Future Microbiol. 2017 Jun;12:655-670.
Ceftazidime-Avibactam is a combination of a third-generation cephalosporin and a novel non-beta-lactam beta-lactamase inhibitor. This combination was recently recommended for the treatment of complicated urinary tract infections, including acute pyelonephritis, in adults with limited or no alternative treatment options. The current review is aimed to determine activity, efficacy and safety of ceftazidime-Avibactam in the treatment of patients with complicated urinary tract infections.
Molecular beta-Lactamase Characterization of Aerobic Gram-Negative Pathogens Recovered from Patients Enrolled in the Ceftazidime-Avibactam Phase 3 Trials for Complicated Intra-abdominal Infections, with Efficacies Analyzed against Susceptible and Resistant Subsets.[Pubmed:28348155]
Antimicrob Agents Chemother. 2017 May 24;61(6). pii: AAC.02447-16.
The correlation of the clinical efficacy of ceftazidime-Avibactam (plus metronidazole) with that of meropenem was evaluated in subjects infected with Gram-negative isolates having characterized beta-lactam resistance mechanisms from the complicated intra-abdominal infection (cIAI) phase 3 clinical trials. Enterobacteriaceae isolates displaying ceftriaxone and/or ceftazidime MIC values of >/=2 mug/ml and Pseudomonas aeruginosa isolates with ceftazidime MIC values of >/=16 mug/ml were characterized for extended-spectrum-beta-lactamase (ESBL) content. Enterobacteriaceae and P. aeruginosa isolates with imipenem and meropenem MIC values of >/=2 and >/=8 mug/ml, respectively, were tested for carbapenemase genes. The primary efficacy endpoint was clinical cure at test of cure (TOC) among the members of the microbiologically modified intention-to-treat (mMITT) population. A total of 14.5% (56/387) and 18.8% (74/394) of patients in the ceftazidime-Avibactam and meropenem arms had isolates that met the MIC screening criteria at the baseline visit, respectively. CTX-M variants alone (29.7%; 41/138) or in combination with OXA-1/30 (35.5%; 49/138), most commonly, blaCTX-M group 1 variants (79/90; 87.8%), represented the beta-lactamases most frequently observed among Enterobacteriaceae isolates. Among the patients infected with pathogens that did not meet the screening criteria, 82.2% showed clinical cure in the ceftazidime-Avibactam group versus 85.9% in the meropenem group. Among patients infected with any pathogens that met the MIC screening criteria, clinical cure rates at TOC were 87.5% and 86.5% for the ceftazidime-Avibactam and meropenem groups, respectively. Ceftazidime-Avibactam had clinical cure rates of 92.5% to 90.5% among patients infected with ESBL- and/or carbapenemase-producing Enterobacteriaceae strains at the baseline visit, while meropenem showed rates of 84.9% to 85.4%. The ceftazidime-Avibactam and meropenem groups had cure rates of 75.0% and 86.7%, respectively, among patients having any pathogens producing AmpC enzymes. The efficacy of ceftazidime-Avibactam was similar to that of meropenem for treatment of cIAI caused by ESBL-producing organisms. (This study has been registered at ClinicalTrials.gov under registration no. NCT01499290 and NCT01500239.).
Exploring the Landscape of Diazabicyclooctane (DBO) Inhibition: Avibactam Inactivation of PER-2 beta-Lactamase.[Pubmed:28348157]
Antimicrob Agents Chemother. 2017 May 24;61(6). pii: AAC.02476-16.
PER beta-lactamases are an emerging family of extended-spectrum beta-lactamases (ESBL) found in Gram-negative bacteria. PER beta-lactamases are unique among class A enzymes as they possess an inverted omega (Omega) loop and extended B3 beta-strand. These singular structural features are hypothesized to contribute to their hydrolytic profile against oxyimino-cephalosporins (e.g., cefotaxime and ceftazidime). Here, we tested the ability of Avibactam (AVI), a novel non-beta-lactam beta-lactamase inhibitor to inactivate PER-2. Interestingly, the PER-2 inhibition constants (i.e., k2/K = 2 x 10(3) +/- 0.1 x 10(3) M(-1) s(-1), where k2 is the rate constant for acylation (carbamylation) and K is the equilibrium constant) that were obtained when AVI was tested were reminiscent of values observed testing the inhibition by AVI of class C and D beta-lactamases (i.e., k2/K range of approximately 10(3) M(-1) s(-1)) and not class A beta-lactamases (i.e., k2/K range, 10(4) to 10(5) M(-1) s(-1)). Once AVI was bound, a stable complex with PER-2 was observed via mass spectrometry (e.g., 31,389 +/- 3 atomic mass units [amu] --> 31,604 +/- 3 amu for 24 h). Molecular modeling of PER-2 with AVI showed that the carbonyl of AVI was located in the oxyanion hole of the beta-lactamase and that the sulfate of AVI formed interactions with the beta-lactam carboxylate binding site of the PER-2 beta-lactamase (R220 and T237). However, hydrophobic patches near the PER-2 active site (by Ser70 and B3-B4 beta-strands) were observed and may affect the binding of necessary catalytic water molecules, thus slowing acylation (k2/K) of AVI onto PER-2. Similar electrostatics and hydrophobicity of the active site were also observed between OXA-48 and PER-2, while CTX-M-15 was more hydrophilic. To demonstrate the ability of AVI to overcome the enhanced cephalosporinase activity of PER-2 beta-lactamase, we tested different beta-lactam-AVI combinations. By lowering MICs to
A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia.[Pubmed:28363526]
Int J Antimicrob Agents. 2017 May;49(5):579-588.
Ceftazidime/Avibactam comprises the broad-spectrum cephalosporin ceftazidime and the non-beta-lactam beta-lactamase inhibitor Avibactam. This phase 3, randomised, double-blind study (NCT01726023) assessed the efficacy and safety of ceftazidime/Avibactam plus metronidazole compared with meropenem in patients with complicated intra-abdominal infection (cIAI) in Asian countries. Subjects aged 18-90 years and hospitalised with cIAI requiring surgical intervention were randomised 1:1 to receive every 8 h either: ceftazidime/Avibactam (2000/500 mg, 2-h infusion) followed by metronidazole (500 mg, 60-min infusion); or meropenem (1000 mg, 30-min infusion). Non-inferiority of ceftazidime/Avibactam plus metronidazole to meropenem was concluded if the lower limit of the 95% confidence interval (CI) for the between-group difference in clinical cure rate was greater than -12.5% at the test-of-cure (TOC) visit (28-35 days after randomisation) in the clinically evaluable (CE) population. Safety was also evaluated. Of 441 subjects randomised, 432 received at least one dose of study medication (ceftazidime/Avibactam plus metronidazole, n = 215; meropenem, n = 217). In the CE population at the TOC visit, non-inferiority of ceftazidime/Avibactam plus metronidazole to meropenem was demonstrated, with clinical cure reported for 93.8% (166/177) and 94.0% (173/184) of subjects, respectively (between-group difference, -0.2, 95% CI -5.53 to 4.97). The clinical cure rate with ceftazidime/Avibactam plus metronidazole was comparable in subjects with ceftazidime-non-susceptible and ceftazidime-susceptible isolates (95.7% vs. 92.1%, respectively). Adverse events were similar between the study groups. Ceftazidime/Avibactam plus metronidazole was non-inferior to meropenem in the treatment of cIAIs in Asian populations and was effective against ceftazidime-non-susceptible pathogens. No new safety concerns were identified.


