10-HydroxyscandineCAS# 119188-47-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 119188-47-5 | SDF | Download SDF |
| PubChem ID | 12082289 | Appearance | Powder |
| Formula | C21H22N2O4 | M.Wt | 366.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
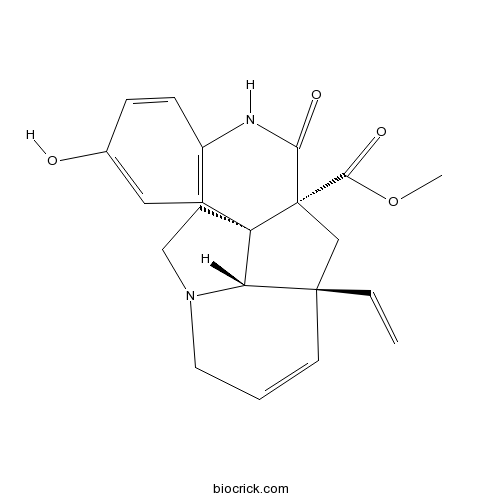
| SMILES | COC(=O)C12CC3(C=CCN4C3C1(CC4)C5=C(C=CC(=C5)O)NC2=O)C=C | ||
| Standard InChIKey | LGEFXJCPQAMQOD-VRXWPRPYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | J Org Chem. 2000 Jun 16;65(12):3804-10.Alkaloid-fullerene systems through photocycloaddition reactions.[Pubmed: 10864768]The photocycloaddition of tertiary amines to ¿60fullerene (C(60)) is an interesting and useful reaction.
Planta Med. 1988 Aug;54(4):315-7.Study on the Alkaloids of Melodinus tenuicaudatus.[Pubmed: 17265274 ]
|

10-Hydroxyscandine Dilution Calculator

10-Hydroxyscandine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7293 mL | 13.6463 mL | 27.2926 mL | 54.5852 mL | 68.2314 mL |
| 5 mM | 0.5459 mL | 2.7293 mL | 5.4585 mL | 10.917 mL | 13.6463 mL |
| 10 mM | 0.2729 mL | 1.3646 mL | 2.7293 mL | 5.4585 mL | 6.8231 mL |
| 50 mM | 0.0546 mL | 0.2729 mL | 0.5459 mL | 1.0917 mL | 1.3646 mL |
| 100 mM | 0.0273 mL | 0.1365 mL | 0.2729 mL | 0.5459 mL | 0.6823 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Coronarin B
Catalog No.:BCN6077
CAS No.:119188-38-4
- Coronarin D
Catalog No.:BCN6076
CAS No.:119188-37-3
- Coronarin A
Catalog No.:BCN6075
CAS No.:119188-33-9
- Glychionide A
Catalog No.:BCN3250
CAS No.:119152-50-0
- 3-Oxosapriparaquinone
Catalog No.:BCN3153
CAS No.:119139-56-9
- B-Raf inhibitor 1 dihydrochloride
Catalog No.:BCC4183
CAS No.:1191385-19-9
- Ganomycin I
Catalog No.:BCN3504
CAS No.:1191255-15-8
- IOWH-032
Catalog No.:BCC3922
CAS No.:1191252-49-9
- 1-Acetoxy-5-deacetylbaccatin I
Catalog No.:BCN6357
CAS No.:119120-27-3
- Linolenic acid ethyl ester
Catalog No.:BCN8333
CAS No.:1191-41-9
- Phellolactone
Catalog No.:BCN3467
CAS No.:1190897-23-4
- Fluorobexarotene
Catalog No.:BCC6110
CAS No.:1190848-23-7
- CZC-25146
Catalog No.:BCC5371
CAS No.:1191911-26-8
- CZC 54252 hydrochloride
Catalog No.:BCC6218
CAS No.:1191911-27-9
- 4-(1H-1,2,4-Triazol-1-ylmethyl)aniline
Catalog No.:BCC8645
CAS No.:119192-10-8
- PHT-427
Catalog No.:BCC2554
CAS No.:1191951-57-1
- LX7101 HCL
Catalog No.:BCC6414
CAS No.:1192189-69-7
- Gomisin S
Catalog No.:BCN3622
CAS No.:119239-49-5
- Avibactam
Catalog No.:BCC1384
CAS No.:1192500-31-4
- Scutellarin methylester
Catalog No.:BCN2828
CAS No.:119262-68-9
- PE 154
Catalog No.:BCC7858
CAS No.:1192750-33-6
- Rocuronium Bromide
Catalog No.:BCC1068
CAS No.:119302-91-9
- Atalantoflavone
Catalog No.:BCN4857
CAS No.:119309-02-3
- Pinobanksin 5-methyl ether
Catalog No.:BCN7775
CAS No.:119309-36-3
Study on the Alkaloids of Melodinus tenuicaudatus.[Pubmed:17265274]
Planta Med. 1988 Aug;54(4):315-7.
Fourteen alkaloids were isolated from the stem bark of MELODINUS TENUICAUDATUS Tsiang et P. T. Li. Eleven of them were identified as known alkaloids, namely, scandine ( 2), Delta (14)-eburnamine ( 4), vindolinine N(b)-oxide ( 5), 11-methoxytabersonine ( 6), vindolinine ( 7), EPI-vindolinine N(b)-oxide ( 8), hazuntine ( 9), compactinervine ( 10), 11-hydroxytabersonine ( 11), Delta (14)-vincine ( 12), and normacusine B ( 14). Two alkaloids were new: 10-Hydroxyscandine ( 1), and the dimer, tenuicausine ( 3); their structures were elucidated by spectroscopic and chemical methods. One alkaloid ( 13) occurring in trace amounts, could not be identified.
Alkaloid-fullerene systems through photocycloaddition reactions.[Pubmed:10864768]
J Org Chem. 2000 Jun 16;65(12):3804-10.
The photocycloaddition of tertiary amines to inverted question mark60fullerene (C(60)) is an interesting and useful reaction. We wished to extend the applications of this type of reaction through an investigation of the photoaddition of alkaloids to C(60) for the purpose of synthesizing novel and complex photoadducts that are difficult to obtain by usual methods. Irradiation of tazettine (2) or gramine (3) with C(60) in toluene leads to formation of one monoadduct (6 or 7), whereas scandine (1a) or 10-Hydroxyscandine (1b) reacts with C(60) photochemically to give two products, the expected inverted question mark6,6 monoadduct (5a, 5b) and a new type of monoadduct with a bis- inverted question mark6, 6 closed structure (4a, 4b). These new structures were characterized by UV-vis, FT-IR, (1)H NMR, (13)C NMR, (1)H-(1)H COSY, ROESY, HMQC (heteronuclear multiple-quantum coherence), and HMBC (heteronuclear multiple-bond connectivity) spectroscopy. The techniques of time-of-flight secondary ion MS (TOF-SIMS) and field desorption MS (FD-MS) were used for the mass determination. (3)He NMR analysis of the product mixture from photoaddition of 1a to C(60) containing a (3)He atom ((3)He@C(60)) led to two peaks at -9.091 and -11.090 ppm relative to gaseous (3)He, consistent with formation of a inverted question mark6, 6-closed monoadduct and a bis- inverted question mark6,6 closed adduct. Presumably, the bis- inverted question mark6, 6 closed adducts are formed by an intramolecular inverted question mark2 + 2 cycloaddition of the vinyl group to the adjacent 6,6-ring junction of C(60) after the initial photocycloaddition.


