PHT-427Akt and PDPK1 inhibitor CAS# 1191951-57-1 |
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- A-443654
Catalog No.:BCC1321
CAS No.:552325-16-3
- AKT inhibitor VIII
Catalog No.:BCC1334
CAS No.:612847-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1191951-57-1 | SDF | Download SDF |
| PubChem ID | 44240850 | Appearance | Powder |
| Formula | C20H31N3O2S2 | M.Wt | 409.61 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (122.07 mM; Need ultrasonic) | ||
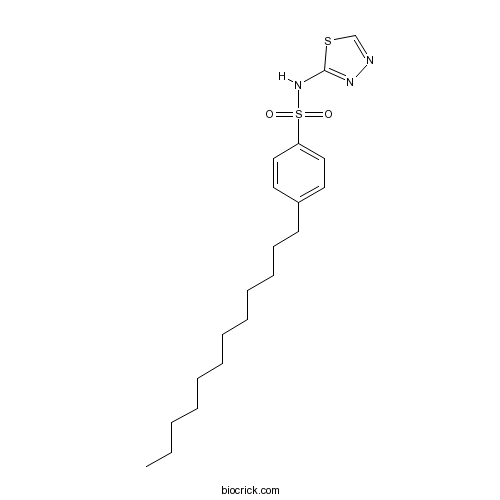
| Chemical Name | 4-dodecyl-N-(1,3,4-thiadiazol-2-yl)benzenesulfonamide | ||
| SMILES | CCCCCCCCCCCCC1=CC=C(C=C1)S(=O)(=O)NC2=NN=CS2 | ||
| Standard InChIKey | BYWWNRBKPCPJMG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H31N3O2S2/c1-2-3-4-5-6-7-8-9-10-11-12-18-13-15-19(16-14-18)27(24,25)23-20-22-21-17-26-20/h13-17H,2-12H2,1H3,(H,22,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dual Akt and PDK1 inhibitor; binds the pleckstrin homology (PH) domain of Akt and PDK1 (Ki values are 2.7 and 5.2 μM, respectively). Inhibits Akt phosphorylation and Akt downstream targets; also induces apoptosis in vitro. Displays synergistic antitumor effect when combined with chemotherapeutics in breast cancer mouse models. |

PHT-427 Dilution Calculator

PHT-427 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4413 mL | 12.2067 mL | 24.4135 mL | 48.8269 mL | 61.0337 mL |
| 5 mM | 0.4883 mL | 2.4413 mL | 4.8827 mL | 9.7654 mL | 12.2067 mL |
| 10 mM | 0.2441 mL | 1.2207 mL | 2.4413 mL | 4.8827 mL | 6.1034 mL |
| 50 mM | 0.0488 mL | 0.2441 mL | 0.4883 mL | 0.9765 mL | 1.2207 mL |
| 100 mM | 0.0244 mL | 0.1221 mL | 0.2441 mL | 0.4883 mL | 0.6103 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PHT-427 is an inhibitor of Akt and PDPK1 (Ki =2.7 μM and 5.2 μM, respectively).
Akt is a serine/threonine-specific protein kinase that plays a vital role in multiple cellular processes including glucose metabolism, apoptosis, cell proliferation, transcription and cell migration etc.
In BxPC-3 cells, PHT-427 showed inhibition upon Akt function with IC50 value of 8.6±0.8 μM and for its downstream substrates. PHT-427 reduced the Akt phosphorylation on Ser473 residue and did not decrease total Akt protein level. PHT-427 also inhibited p70S6K and GSK3β in a dose-dependent manner. [1][2]
In SCID (severe combined immunodeficiency) mice of BxPC-3 pancreatic cancer xenografts, administration of PHT-427 exerted prominent antitumor activity that halted tumor growth. PHT-427 in combination with erlotinib exhibited greater than additive antitumor activity in NSC lung cancer and with paclitaxel in breast cancer. [1][2]
References:
1. Meuillet EJ, Zuohe S, Lemos R et al. Molecular pharmacology and antitumor activity of PHT-427, a novel Akt/phosphatidylinositide-dependent protein kinase 1 pleckstrin homology domain inhibitor. Mol Cancer Ther. 2010 Mar;9(3):706-17.
2. Moses SA, Ali MA, Zuohe S et al. In vitro and in vivo activity of novel small-molecule inhibitors targeting the pleckstrin homology domain of protein kinase B/AKT. Cancer Res. 2009 Jun 15;69(12):5073-81.
- 4-(1H-1,2,4-Triazol-1-ylmethyl)aniline
Catalog No.:BCC8645
CAS No.:119192-10-8
- CZC 54252 hydrochloride
Catalog No.:BCC6218
CAS No.:1191911-27-9
- CZC-25146
Catalog No.:BCC5371
CAS No.:1191911-26-8
- 10-Hydroxyscandine
Catalog No.:BCN6078
CAS No.:119188-47-5
- Coronarin B
Catalog No.:BCN6077
CAS No.:119188-38-4
- Coronarin D
Catalog No.:BCN6076
CAS No.:119188-37-3
- Coronarin A
Catalog No.:BCN6075
CAS No.:119188-33-9
- Glychionide A
Catalog No.:BCN3250
CAS No.:119152-50-0
- 3-Oxosapriparaquinone
Catalog No.:BCN3153
CAS No.:119139-56-9
- B-Raf inhibitor 1 dihydrochloride
Catalog No.:BCC4183
CAS No.:1191385-19-9
- Ganomycin I
Catalog No.:BCN3504
CAS No.:1191255-15-8
- IOWH-032
Catalog No.:BCC3922
CAS No.:1191252-49-9
- LX7101 HCL
Catalog No.:BCC6414
CAS No.:1192189-69-7
- Gomisin S
Catalog No.:BCN3622
CAS No.:119239-49-5
- Avibactam
Catalog No.:BCC1384
CAS No.:1192500-31-4
- Scutellarin methylester
Catalog No.:BCN2828
CAS No.:119262-68-9
- PE 154
Catalog No.:BCC7858
CAS No.:1192750-33-6
- Rocuronium Bromide
Catalog No.:BCC1068
CAS No.:119302-91-9
- Atalantoflavone
Catalog No.:BCN4857
CAS No.:119309-02-3
- Pinobanksin 5-methyl ether
Catalog No.:BCN7775
CAS No.:119309-36-3
- 2-Benzyl-2-(dimethylamino)-4'-morpholinobutyrophenone
Catalog No.:BCC8563
CAS No.:119313-12-1
- Olean-12-ene-3,24-diol
Catalog No.:BCN6079
CAS No.:119318-15-9
- 2-Hydroxydiplopterol
Catalog No.:BCN7290
CAS No.:1193250-54-2
- CRANAD 2
Catalog No.:BCC6293
CAS No.:1193447-34-5
Molecular pharmacology and antitumor activity of PHT-427, a novel Akt/phosphatidylinositide-dependent protein kinase 1 pleckstrin homology domain inhibitor.[Pubmed:20197390]
Mol Cancer Ther. 2010 Mar;9(3):706-17.
Phosphatidylinositol 3-kinase/phosphatidylinositide-dependent protein kinase 1 (PDPK1)/Akt signaling plays a critical role in activating proliferation and survival pathways within cancer cells. We report the molecular pharmacology and antitumor activity of PHT-427, a compound designed to bind to the pleckstrin homology (PH) binding domain of signaling molecules important in cancer. Although originally designed to bind the PH domain of Akt, we now report that PHT-427 also binds to the PH domain of PDPK1. A series of PHT-427 analogues with variable C-4 to C-16 alkyl chain length were synthesized and tested. PHT-427 itself (C-12 chain) bound with the highest affinity to the PH domains of both PDPK1 and Akt. PHT-427 inhibited Akt and PDPK1 signaling and their downstream targets in sensitive but not resistant cells and tumor xenografts. When given orally, PHT-427 inhibited the growth of human tumor xenografts in immunodeficient mice, with up to 80% inhibition in the most sensitive tumors, and showed greater activity than analogues with C4, C6, or C8 alkyl chains. Inhibition of PDPK1 was more closely correlated to antitumor activity than Akt inhibition. Tumors with PIK3CA mutation were the most sensitive, and K-Ras mutant tumors were the least sensitive. Combination studies showed that PHT-427 has greater than additive antitumor activity with paclitaxel in breast cancer and with erlotinib in non-small cell lung cancer. When given >5 days, PHT-427 caused no weight loss or change in blood chemistry. Thus, we report a novel PH domain binding inhibitor of PDPK1/Akt signaling with significant in vivo antitumor activity and minimal toxicity.
In vitro and in vivo activity of novel small-molecule inhibitors targeting the pleckstrin homology domain of protein kinase B/AKT.[Pubmed:19491272]
Cancer Res. 2009 Jun 15;69(12):5073-81.
The phosphatidylinositol 3-kinase/AKT signaling pathway plays a critical role in activating survival and antiapoptotic pathways within cancer cells. Several studies have shown that this pathway is constitutively activated in many different cancer types. The goal of this study was to discover novel compounds that bind to the pleckstrin homology (PH) domain of AKT, thereby inhibiting AKT activation. Using proprietary docking software, 22 potential PH domain inhibitors were identified. Surface plasmon resonance spectroscopy was used to measure the binding of the compounds to the expressed PH domain of AKT followed by an in vitro activity screen in Panc-1 and MiaPaCa-2 pancreatic cancer cell lines. We identified a novel chemical scaffold in several of the compounds that binds selectively to the PH domain of AKT, inducing a decrease in AKT activation and causing apoptosis at low micromolar concentrations. Structural modifications of the scaffold led to compounds with enhanced inhibitory activity in cells. One compound, 4-dodecyl-N-(1,3,4-thiadiazol-2-yl)benzenesulfonamide, inhibited AKT and its downstream targets in cells as well as in pancreatic cancer cell xenografts in immunocompromised mice; it also exhibited good antitumor activity. In summary, a pharmacophore for PH domain inhibitors targeting AKT function was developed. Computer-aided modeling, synthesis, and testing produced novel AKT PH domain inhibitors that exhibit promising preclinical properties.


