PerindoprilACE inhibitor CAS# 82834-16-0 |
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
- Irbesartan
Catalog No.:BCC2560
CAS No.:138402-11-6
- Olmesartan
Catalog No.:BCC1819
CAS No.:144689-24-7
- Tranilast
Catalog No.:BCC2514
CAS No.:53902-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 82834-16-0 | SDF | Download SDF |
| PubChem ID | 107807 | Appearance | Powder |
| Formula | C19H32N2O5 | M.Wt | 368.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
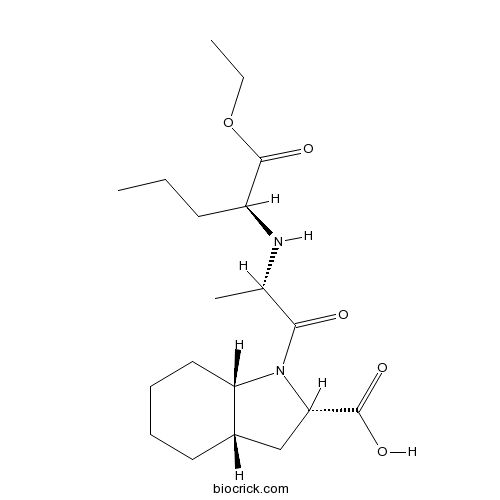
| Chemical Name | (2S,3aS,7aS)-1-[(2S)-2-[[(2S)-1-ethoxy-1-oxopentan-2-yl]amino]propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carboxylic acid | ||
| SMILES | CCCC(C(=O)OCC)NC(C)C(=O)N1C2CCCCC2CC1C(=O)O | ||
| Standard InChIKey | IPVQLZZIHOAWMC-QXKUPLGCSA-N | ||
| Standard InChI | InChI=1S/C19H32N2O5/c1-4-8-14(19(25)26-5-2)20-12(3)17(22)21-15-10-7-6-9-13(15)11-16(21)18(23)24/h12-16,20H,4-11H2,1-3H3,(H,23,24)/t12-,13-,14-,15-,16-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Perindopril Dilution Calculator

Perindopril Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7139 mL | 13.5696 mL | 27.1393 mL | 54.2785 mL | 67.8481 mL |
| 5 mM | 0.5428 mL | 2.7139 mL | 5.4279 mL | 10.8557 mL | 13.5696 mL |
| 10 mM | 0.2714 mL | 1.357 mL | 2.7139 mL | 5.4279 mL | 6.7848 mL |
| 50 mM | 0.0543 mL | 0.2714 mL | 0.5428 mL | 1.0856 mL | 1.357 mL |
| 100 mM | 0.0271 mL | 0.1357 mL | 0.2714 mL | 0.5428 mL | 0.6785 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Perindopril is an inhibitor of angiotensin-I converting enzyme (ACE) [1].
As an inhibitor of ACE, perindopril has been reported to have anticancer activity through the inhibition of tumor growth and angiogenesis in hepatocellular carcinoma cells. In the in vitro cell proliferation assays, perindopril does not show significant effect on the cell proliferation. However, even 1μM perindoprilat can significantly reduce mRNA expression of VEGF and can inhibit the induction activity of the VEGF promoter in KB cells. Also in KB cells, 1μM perindoprilat treatment significantly suppresses the angiotensin II production. Besides that, In vivo assays demonstrate perindopril can suppress the growth of SCC-VII cells [1]. Moreover, it is reported that 8 mg perindopril given orally can lower blood pressure without a change in heart rate [1, 2].
References:
[1] Ryuji Yasumatsu, Torahiko Nakashima, Muneyuki Masuda, Aya Ito, Yuichiro Kuratomi, Yuichiro Kuratomi and Shizuo Komune. Effects of the angiotensin-I converting enzyme inhibitor perindopril on tumor growth and angiogenesis in head and neck squamous cell carcinoma cells. J Cancer Res Clin Oncol. 2004, 130: 567–573.
[2] A.A.Ajayi, K.R.Lees and J.L.Reid. Effects of angiotensin converting enzyme inhibitor, perindopril, on autonomic reflexes. Eur J Clin Pharmacol. 1986, 30:177-182.
- cGMP Dependent Kinase Inhibitor Peptide
Catalog No.:BCC8084
CAS No.:82801-73-8
- H-D-HoPhe-OH
Catalog No.:BCC3239
CAS No.:82795-51-5
- Nefazodone hydrochloride
Catalog No.:BCC7479
CAS No.:82752-99-6
- DCPIB
Catalog No.:BCC7105
CAS No.:82749-70-0
- 6,7-Dihydroxy-4-(Trifluoromethyl)Coumarin
Catalog No.:BCC8286
CAS No.:82747-36-2
- Perforatumone
Catalog No.:BCN4370
CAS No.:827319-50-6
- Danusertib (PHA-739358)
Catalog No.:BCC2172
CAS No.:827318-97-8
- Palbociclib (PD0332991) Isethionate
Catalog No.:BCC3698
CAS No.:827022-33-3
- PD 0332991 (Palbociclib) HCl
Catalog No.:BCC3680
CAS No.:827022-32-2
- Boc-Tryptophanol
Catalog No.:BCC2700
CAS No.:82689-19-8
- Picrasidine A
Catalog No.:BCN4369
CAS No.:82652-20-8
- Raloxifene HCl
Catalog No.:BCC4488
CAS No.:82640-04-8
- Pingpeimine A
Catalog No.:BCN8407
CAS No.:82841-67-6
- Pingpeimine B
Catalog No.:BCN8408
CAS No.:82851-52-3
- Echinacoside
Catalog No.:BCN4953
CAS No.:82854-37-3
- Cyclo(Tyr-Leu)
Catalog No.:BCN2432
CAS No.:82863-65-8
- 2,3-Dihydroisoginkgetin
Catalog No.:BCN4035
CAS No.:828923-27-9
- (R)-(+)-Etomoxir sodium salt
Catalog No.:BCC7946
CAS No.:828934-41-4
- 3-Oxo-24,25,26,27-tetranortirucall-7-en-23,21-olide
Catalog No.:BCN1338
CAS No.:828935-47-3
- Fmoc-Osu
Catalog No.:BCC2804
CAS No.:82911-69-1
- 7,4'-Dihydroxy-8-methylflavan
Catalog No.:BCN6841
CAS No.:82925-55-1
- CI 898 trihydrochloride
Catalog No.:BCC7248
CAS No.:82952-64-5
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
- 11-O-Galloylbergenin
Catalog No.:BCN6637
CAS No.:82958-44-9
[Evaluation of Antihypertensive Efficacy and Patient Adherence to Treatment With the New Formulation Perindopril Arginine (Orally Disintegrating Tablet) in General Clinical Practice: OPTIMUM Program].[Pubmed:28294857]
Kardiologiia. 2016 Apr;56(4):36-41.
The purpose of this study was to evaluate the antihypertensive efficacy of Perindopril arginine in the oral dispersible form of tablets, in clinical practice. MATERIALS AND METHODS: Post-marketing observational open program OPTIMUM was carried out from october 2014 to march 2015 year in 48 cities of the Russian Federation, 197 doctors. The observation included 957 patients. Prematurely retired from the study 17patients completed the observation in accordance with the protocol of 940 people. RESULTS: Of the patients included men was 40.8%, 28.1% smokers , obese patients were 32.2% , cholesterol >5.0 mmol/l - 54.1%, diabetes - 13.3% . Angina was at 19.5% of patients had previous myocardial infarction, 5.3%, 3.7% of stroke, heart failure, was at 26.5% . Previously treated with 16% of patients , 53% had received monotherapy 2-koponentnuyu - 35% 3-component therapy and more 13% of patients. Angiotensin converting enzyme obtained 50.9% of the patients, 20.2% Sartana, diuretics - 22.9%, -blockers - 25.8%, calcium antagonists - 13.6% other drugs - 2.2%. With the inclusion in the program every fifth patient had BP Stage I level and included patients had hypertension II degree. The mean duration of disease was 7.0+/-6.2 years (median 5 years). When replacing the previously used ACE inhibitors or sartans on mouth-dispersible form of Perindopril arginine BP decreased from 140,9+/-12.7/84.2+/-7.6 to 127.0+/-8.4/78.1+/-5.8 mm Hg. Target BP <140/90 mm Hg 91.4% of patients reached after 3 months of treatment. Adherence to the treatment of patients improved significantly and the total score increased from an average of 2.76+/-1.25 to 3.57+/-0.89 points (p<0.00001). When filling out a special questionnaire >2/3 of patients agreed or strongly agreed with the statements that orodispersible form is more easy to use, dissolves quickly and easily swallowed, is more convenient for the reception is at home or traveling. CONCLUSION: It can be expected that the extension of the possibility of using Perindopril arginine, including through the appointment of a new form for the reception, can lead to increased adherence to treatment, improve blood pressure control and reduce the incidence of adverse events in patients with hypertension.
[Effect of Combined Antihypertensive Therapy With Perindopril and Indapamide on Morpho-Functional Parameters of The Heart, Blood Vessels of Small and Medium Caliber in Patients With Essential Hypertension].[Pubmed:28294884]
Kardiologiia. 2016 Mar;56(3):19-24.
AIM: to study effect of therapy with fixed combination of angiotensin-converting enzyme infibitor and diuretic on structural-functional parameters of the heart and vessels and cognitive function. MATERIAL AND METHODS: We included into this study 30 patients (20 women) with mean age 60.06+/-10.19 years, duration of hypertensive disease 14.7 (3; 32) years, body mass index 31.19+/-3.93 kg/m2, and without history of cerebrovascular diseases. Methods of investigation included clinical examination, measurement of parameters of hemodynamics, electro- and echocardiography. Vascular endothelial function and structural - functional state of finger skin capillary network was studied by photopletismography and video capillaroscopy, respectively. The state of cognitive sphere was evaluated with the help of the Montreal Cognitive Assessment (MoCA) test. RESULTS: We noted improvement of endothelial function of middle caliber arteries and microcirculatory vascular bed (MCVB) (increase of occlusion index at the level of MCVB from 1.4 to 1.8, p<0.00005; increase of density of skin capillary network at rest from 45 to 52 kap/2, p<0.00007); improvement of cognitive function according to changes of MoCA test results (from 23 to 27, p<0,0001). There were no changes of lipid and carbohydrate metabolism, and electrolyte balance. Conclision. The use of fixed of Perindopril+indopamide is combination characterized by good tolerability and high results.
Blood pressure-lowering efficacy and safety of perindopril/indapamide/amlodipine single-pill combination in patients with uncontrolled essential hypertension: a multicenter, randomized, double-blind, controlled trial.[Pubmed:28306636]
J Hypertens. 2017 Jul;35(7):1481-1495.
OBJECTIVES: This 4-month, double-blind, randomized, controlled trial was designed to demonstrate the superiority of Perindopril/indapamide/amlodipine single pill over Perindopril/indapamide after 1 month and to determine further up-titration efficacy and safety in patients with mild-to-moderate hypertension. METHODS: After a 1-month run-in period on Perindopril/indapamide 5/1.25 mg, patients with SBP/DBP at least 150/95 mmHg and no diabetes or renal insufficiency received Perindopril/indapamide/amlodipine 5/1.25/5 mg single pill or continued on the same treatment. At 1, 2, and 3 months, patients with uncontrolled blood pressure (SBP/DBP >/= 140/90 mmHg) were gradually up-titrated with a higher dose of the triple therapy up to Perindopril/indapamide/amlodipine 10/2.5/10 mg in both groups. Efficacy was assessed on office supine SBP (main criterion) and DBP, blood pressure control, and response rates. Treatment effect on ambulatory blood pressure monitoring (ABPM) and home blood pressure monitoring (HBPM) parameters was also assessed in two subpopulations of 276 and 263 patients, respectively. RESULTS: A total of 454 hypertensive patients (diabetes and renal insufficiency excluded) were randomized, 227 to each group (56% were men, mean age was 55 years, blood pressure 162.3/101.1 mmHg). After 1 month, superior SBP (-3.1 mmHg, P = 0.02) and DBP (-2.8 mmHg, P < 0.001) reductions were observed with Perindopril/indapamide/amlodipine, which were even more pronounced after excluding white-coat effect in the sustained hypertension population (-5.3/-3.7 mmHg). Similar results were observed in terms of blood pressure response (72 vs. 53%, P < 0.0001) and control rates (32 vs. 25%, P = 0.005). Up-titration was effective at each visit in both treatment arms (P < 0.001). Both ABPM and HBPM results confirmed the superiority of the triple therapy at 1 month on ASBP/ADBP and HSBP/HDBP: -4.5/-2.0 mmHg for ABPM (P < 0.001/P = 0.04), and -4.9/-3.1 mmHg for HBPM (both, P < 0.001). Up-titration steps resulted in further significant decreases in both ABPM and HBPM. Both treatment regimens were well tolerated regarding adverse events or laboratory testing. In particular, peripheral edema known to be amlodipine dose dependent, appeared in only a few cases, none with the highest dose. Hypotension, orthostatic hypotension, and cough whatever the dose were infrequent. There were no treatment-related serious adverse events. CONCLUSION: Perindopril/indapamide/amlodipine in a single pill produces superior reductions in blood pressure compared with dual therapy. Triple therapy up-titration was well tolerated and effective leading to BP control rates of over 80%. Analysis of 24-h ABPM and HBPM results corroborated these findings.
[Efficacy and Safety of a Fixed Combination of Perindopril Arginine and Amlodipine in Patients With Hypertension Uncontrolled by Treatment With Angiotensin II Receptor Blockers in Real Clinical Practice. Results of the PREVOSHODSTVO (SUPERIORITY) Program].[Pubmed:28290831]
Kardiologiia. 2017 Jan;(1):30-36.
The article presents preliminary results of a subanalysis of PREVOSHODSTVO (SUPERIORITY) phase IV study. Aim of this subanalysis was to assess efficacy and tolerability of a fixed-dose Perindopril/amlodipine combination (FDPAC) in patients with arterial hypertension (AP) uncontrolled on previous treatment with angiotensin receptor blockers (ARBs). MATERIAL AND METHODS: We included in this analysis 125 patients (70.4% women, mean age 57.2+/-10.0 years), final analysis of efficacy was performed on 124 patients. Before inclusion in the study 47 patients received ARB either as monotherapy (n=47), or components of free-dose (n=49) and fixed-dose (n=28) dual combinations with other antihypertensive drugs. Dose. of FDPAC was determined by physician. Duration of observation period was 24 weeks. RESULTS: After 2weeks significant reduction of blood pressure (BP) (from 159.9+/-8.8/93.8+/-6.8 to 143.9+/-10.7/86.4+/-6.5 mm Hg, p<0.001) was noted. At final visit mean BP was 125.1+/-7.1/78.1+/-4.7 mm Hg. Number of patients with target BP (< 140/90 mm Hg) was 24, 75 and 97% after 1, 3, and 6 months, respectively. Visit-to-visit systolic BP variability by the end of the observation period decreased to 3.8+/-2.3 mm Hg. CONCLUSION: In patients, whose hypertension was not controlled by treatment with ARBs the fixed-dose combination of Perindopril/amlodipine provided high percentage of achievement of target BP and reduction of long-term BP variability.


