Nefazodone hydrochloride5-HT uptake inhibitor and 5-HT2A antagonist. Antidepressant CAS# 82752-99-6 |
- Dexmedetomidine HCl
Catalog No.:BCC4347
CAS No.:145108-58-3
- Xylazine HCl
Catalog No.:BCC4341
CAS No.:23076-35-9
- Guanfacine
Catalog No.:BCC5180
CAS No.:29110-47-2
- Sotalol
Catalog No.:BCC4356
CAS No.:3930-20-9
- Isoprenaline HCl
Catalog No.:BCC4328
CAS No.:51-30-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 82752-99-6 | SDF | Download SDF |
| PubChem ID | 54911 | Appearance | Powder |
| Formula | C25H33Cl2N5O2 | M.Wt | 506.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (98.72 mM; Need ultrasonic) H2O : 2 mg/mL (3.95 mM; Need ultrasonic) | ||
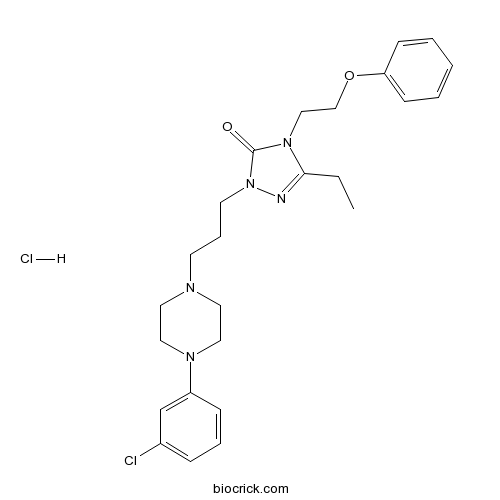
| Chemical Name | 2-[3-[4-(3-chlorophenyl)piperazin-1-yl]propyl]-5-ethyl-4-(2-phenoxyethyl)-1,2,4-triazol-3-one;hydrochloride | ||
| SMILES | CCC1=NN(C(=O)N1CCOC2=CC=CC=C2)CCCN3CCN(CC3)C4=CC(=CC=C4)Cl.Cl | ||
| Standard InChIKey | DYCKFEBIOUQECE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H32ClN5O2.ClH/c1-2-24-27-31(25(32)30(24)18-19-33-23-10-4-3-5-11-23)13-7-12-28-14-16-29(17-15-28)22-9-6-8-21(26)20-22;/h3-6,8-11,20H,2,7,12-19H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Serotonin 5-HT2A receptor antagonist (Ki = 5.8 nM) and inhibitor of serotonin and noradrenalin uptake (IC50 values are 290 and 300 nM respectively). Displays no activity at 5-HT1B and 5-HT1D receptors. Active in models predictive of antidepressant potential. |

Nefazodone hydrochloride Dilution Calculator

Nefazodone hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9745 mL | 9.8723 mL | 19.7445 mL | 39.489 mL | 49.3613 mL |
| 5 mM | 0.3949 mL | 1.9745 mL | 3.9489 mL | 7.8978 mL | 9.8723 mL |
| 10 mM | 0.1974 mL | 0.9872 mL | 1.9745 mL | 3.9489 mL | 4.9361 mL |
| 50 mM | 0.0395 mL | 0.1974 mL | 0.3949 mL | 0.7898 mL | 0.9872 mL |
| 100 mM | 0.0197 mL | 0.0987 mL | 0.1974 mL | 0.3949 mL | 0.4936 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- DCPIB
Catalog No.:BCC7105
CAS No.:82749-70-0
- 6,7-Dihydroxy-4-(Trifluoromethyl)Coumarin
Catalog No.:BCC8286
CAS No.:82747-36-2
- Perforatumone
Catalog No.:BCN4370
CAS No.:827319-50-6
- Danusertib (PHA-739358)
Catalog No.:BCC2172
CAS No.:827318-97-8
- Palbociclib (PD0332991) Isethionate
Catalog No.:BCC3698
CAS No.:827022-33-3
- PD 0332991 (Palbociclib) HCl
Catalog No.:BCC3680
CAS No.:827022-32-2
- Boc-Tryptophanol
Catalog No.:BCC2700
CAS No.:82689-19-8
- Picrasidine A
Catalog No.:BCN4369
CAS No.:82652-20-8
- Raloxifene HCl
Catalog No.:BCC4488
CAS No.:82640-04-8
- Zolpidem
Catalog No.:BCC9194
CAS No.:82626-48-0
- Pseudolaric Acid C
Catalog No.:BCN2856
CAS No.:82601-41-0
- Mecamylamine hydrochloride
Catalog No.:BCC7501
CAS No.:826-39-1
- H-D-HoPhe-OH
Catalog No.:BCC3239
CAS No.:82795-51-5
- cGMP Dependent Kinase Inhibitor Peptide
Catalog No.:BCC8084
CAS No.:82801-73-8
- Perindopril
Catalog No.:BCC4223
CAS No.:82834-16-0
- Pingpeimine A
Catalog No.:BCN8407
CAS No.:82841-67-6
- Pingpeimine B
Catalog No.:BCN8408
CAS No.:82851-52-3
- Echinacoside
Catalog No.:BCN4953
CAS No.:82854-37-3
- Cyclo(Tyr-Leu)
Catalog No.:BCN2432
CAS No.:82863-65-8
- 2,3-Dihydroisoginkgetin
Catalog No.:BCN4035
CAS No.:828923-27-9
- (R)-(+)-Etomoxir sodium salt
Catalog No.:BCC7946
CAS No.:828934-41-4
- 3-Oxo-24,25,26,27-tetranortirucall-7-en-23,21-olide
Catalog No.:BCN1338
CAS No.:828935-47-3
- Fmoc-Osu
Catalog No.:BCC2804
CAS No.:82911-69-1
- 7,4'-Dihydroxy-8-methylflavan
Catalog No.:BCN6841
CAS No.:82925-55-1
Evaluation of sleep architecture and cyclic alternating pattern rates in depressed insomniac patients treated with nefazodone hydrochloride.[Pubmed:10423649]
Am J Ther. 1999 Mar;6(2):77-82.
The standard methods of scoring sleep patterns do not ensure an accurate clinical impression of sleep quality. This is important especially in depressed insomniacs because persistent poor sleep increases the likelihood of recurrent depressive episodes. Changes in cyclic alternating patterns (CAP) in sleep have been shown to reflect corresponding changes in sleep quality. We evaluated the effects of nefazodone on CAP and standard sleep architecture in depressed insomniacs. The study was a single-center, single-blind, 6-week treatment of Nefazodone hydrochloride followed by placebo withdrawal in 16 subjects meeting the DSM-IV criteria for depression who had a score of at least 18 on the 17-item Hamilton Depression Rating Scale, with insomnia-related items 4, 5, and 6 having a total score of 3 or greater. A mean daily dose of 339.1 +/- 141.7 mg at endpoint of nefazodone significantly reduced Hamilton Depression Scores from 21.7 +/- 3.0 on baseline to 5.8 +/- 5.3 (P <.05) by the end of the study. Polysomnography showed an improvement in sleep latency and sleep efficiency (P <.05), but no alterations in rapid-eye-movement or slow-wave sleep. Subjective estimates of sleep quality improved throughout the study, but CAP rates did not show a significant improvement. The disparity between CAP rates and sleep quality in depressed insomniacs is discussed.
Nefazodone. A review of its pharmacology and clinical efficacy in the management of major depression.[Pubmed:9098663]
Drugs. 1997 Apr;53(4):608-36.
Nefazodone hydrochloride is a phenylpiperazine antidepressant with a mechanism of action that is distinct from those of other currently available drugs. It potently and selectively blocks postsynaptic serotonin (5-hydroxytryptamine; 5-HT) 5-HT2A receptors and moderately inhibits serotonin and noradrenaline (norepinephrine) reuptake. In short term clinical trials of 6 or 8 weeks' duration, nefazodone produced clinical improvements that were significantly greater than those with placebo and similar to those achieved with imipramine, and the selective serotonin reuptake inhibitors (SSRIs) fluoxetine, paroxetine and sertraline. The optimum therapeutic dosage of nefazodone appears to be between 300 and 600 mg/day. Limited long term data suggest that nefazodone is effective in preventing relapse of depression in patients treated for up to 1 year. Analyses of pooled clinical trial results indicate that nefazodone and imipramine produces similar and significant improvements on anxiety- and agitation-related rating scales compared with placebo in patients with major depression. Short term tolerability data indicate that nefazodone has a lower incidence of adverse anticholinergic, antihistaminergic and adrenergic effects than imipramine. Compared with SSRIs, nefazodone causes fewer activating symptoms, adverse gastrointestinal effects (nausea, diarrhoea, anorexia) and adverse effects on sexual function, but is associated with more dizziness, dry mouth, constipation, visual disturbances and confusion. Available data also suggest that nefazodone is not associated with abnormal weight gain, seizures, priapism or significant sleep disruption, and appears to be relatively safe in overdosage. Nefazodone inhibits the cytochrome P450 3A4 isoenzyme and thus has the potential to interact with a number of drugs. Further long term and comparative studies will provide a more accurate assessment of the relative place of nefazodone in the management of major depression. Nonetheless, available data suggest that nefazodone is a worthwhile treatment alternative to tricyclic antidepressants and SSRIs in patients with major depression.
Nefazodone: preclinical pharmacology of a new antidepressant.[Pubmed:2274630]
Psychopharmacol Bull. 1990;26(3):311-5.
Recent pharmacologic studies suggest that nefazodone may possess antidepressant activity. Nefazodone is active in behavioral models predictive of antidepressant potential. It is active in reversing learned helplessness, prevents reserpine-induced ptosis, and enhances response efficiency in the differential reinforcement for low rates of response paradigm. In in vitro studies, nefazodone inhibits the binding of [3H]ketanserin to cortical serotonin2 (5-HT2) binding sites, whereas in vivo, it antagonizes the 5-HT2-mediated quipazine-induced head shake in rats. In ex vivo studies, acute oral administration of nefazodone inhibits cortical serotonin uptake and occupies frontal cortical 5-HT2 receptor binding sites. Chronic administration of nefazodone produces a reduction in 5-HT2-mediated behavior and decreases cortical 5-HT2 receptor binding site density. Further, a chronic high-dose nefazodone regimen significantly potentiates 5-HT1A-mediated behavioral responses in rats. Nefazodone exhibits decreased anticholinergic, alpha-adrenolytic, and sedative activity relative to other antidepressants.


