Danusertib (PHA-739358)Pan-aurora kinase inhibitor CAS# 827318-97-8 |
- LY2874455
Catalog No.:BCC1723
CAS No.:1254473-64-7
- SU 5402
Catalog No.:BCC1970
CAS No.:215543-92-3
- PD 173074
Catalog No.:BCC3662
CAS No.:219580-11-7
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
- BGJ398
Catalog No.:BCC1278
CAS No.:872511-34-7
- BIBF 1202
Catalog No.:BCC5298
CAS No.:894783-71-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 827318-97-8 | SDF | Download SDF |
| PubChem ID | 11442891 | Appearance | Powder |
| Formula | C26H30N6O3 | M.Wt | 474.55 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | PHA-739358 | ||
| Solubility | DMSO : 50 mg/mL (105.36 mM; Need ultrasonic) | ||
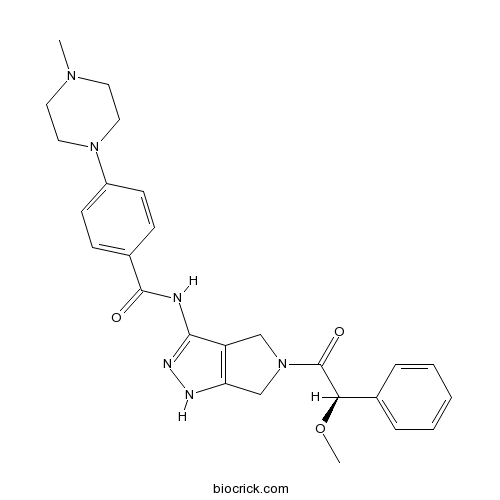
| Chemical Name | N-[5-[(2R)-2-methoxy-2-phenylacetyl]-4,6-dihydro-1H-pyrrolo[3,4-c]pyrazol-3-yl]-4-(4-methylpiperazin-1-yl)benzamide | ||
| SMILES | CN1CCN(CC1)C2=CC=C(C=C2)C(=O)NC3=NNC4=C3CN(C4)C(=O)C(C5=CC=CC=C5)OC | ||
| Standard InChIKey | XKFTZKGMDDZMJI-HSZRJFAPSA-N | ||
| Standard InChI | InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Danusertib (PHA-739358) is an inhibitor of Aurora kinase for Aurora A/B/C with IC50 of 13 nM/79 nM/61 nM, modestly potent to Abl, TrkA, c-RET and FGFR1, and less potent to Lck, VEGFR2/3, c-Kit, CDK2, etc | ||||||
| Targets | Aurora A/B/C | Bcr-Abl | c-RET | FGFR | TrkA | ||
| IC50 | 13 nM/79 nM/61 nM | 25nM | 31 nM | 47 nM | 30 nM | ||

Danusertib (PHA-739358) Dilution Calculator

Danusertib (PHA-739358) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1073 mL | 10.5363 mL | 21.0726 mL | 42.1452 mL | 52.6815 mL |
| 5 mM | 0.4215 mL | 2.1073 mL | 4.2145 mL | 8.429 mL | 10.5363 mL |
| 10 mM | 0.2107 mL | 1.0536 mL | 2.1073 mL | 4.2145 mL | 5.2681 mL |
| 50 mM | 0.0421 mL | 0.2107 mL | 0.4215 mL | 0.8429 mL | 1.0536 mL |
| 100 mM | 0.0211 mL | 0.1054 mL | 0.2107 mL | 0.4215 mL | 0.5268 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Danusertib (previously known as PHA-739358), a 3-aminopyrazole derivative identified during the development of the pyrrolopyrazole sub-series, is a potent small-molecule inhibitor of aurora kinases family members with a dominant inhibition for aurora B kinase (ABK). This pan-aurora kinases inhibitor is also able to inhibit several tyrosine kinases, including T315I mutant, Ret, Trk-A and fibroblast growth factor receptor-1 (FGFR-1), which are involved in multiple malignancies, such as chronic myelogenous leukemia (CML), acute lymphoblastic leukemia (ALL), thyroid prostate and breast carcinoma. Thus, in many previous studies, danusertib exhibits remarkable antitumor activity in a number of different xengorafts, spontaneous, and transgenic animal tumor models with a favorable pharmacokinetic and safety profile.
Reference
Carpinelli P, Ceruti R, Giorgini ML, Cappella P, Gianellini L, Croci V, Degrassi A, Texido G, Rocchetti M, Vianello P, Rusconi L, Storici P, Zugnoni P, Arrigoni C, Soncini C, Alli C, Patton V, Marsiglio A, Ballinari D, Pesenti E, Fancelli D, Moll J. PHA-739358, a potent inhibitor of aurora kinases with a selecyive target inhibiton profile relevant to cancer. Mol Cancer Ther 2007; 6(12 Pt 1): 3158-3168
- Palbociclib (PD0332991) Isethionate
Catalog No.:BCC3698
CAS No.:827022-33-3
- PD 0332991 (Palbociclib) HCl
Catalog No.:BCC3680
CAS No.:827022-32-2
- Boc-Tryptophanol
Catalog No.:BCC2700
CAS No.:82689-19-8
- Picrasidine A
Catalog No.:BCN4369
CAS No.:82652-20-8
- Raloxifene HCl
Catalog No.:BCC4488
CAS No.:82640-04-8
- Zolpidem
Catalog No.:BCC9194
CAS No.:82626-48-0
- Pseudolaric Acid C
Catalog No.:BCN2856
CAS No.:82601-41-0
- Mecamylamine hydrochloride
Catalog No.:BCC7501
CAS No.:826-39-1
- Triacetonamine
Catalog No.:BCN4368
CAS No.:826-36-8
- Sarsaponin
Catalog No.:BCN8293
CAS No.:82597-74-8
- Quinapril HCl
Catalog No.:BCC5011
CAS No.:82586-55-8
- Moexipril HCl
Catalog No.:BCC5015
CAS No.:82586-52-5
- Perforatumone
Catalog No.:BCN4370
CAS No.:827319-50-6
- 6,7-Dihydroxy-4-(Trifluoromethyl)Coumarin
Catalog No.:BCC8286
CAS No.:82747-36-2
- DCPIB
Catalog No.:BCC7105
CAS No.:82749-70-0
- Nefazodone hydrochloride
Catalog No.:BCC7479
CAS No.:82752-99-6
- H-D-HoPhe-OH
Catalog No.:BCC3239
CAS No.:82795-51-5
- cGMP Dependent Kinase Inhibitor Peptide
Catalog No.:BCC8084
CAS No.:82801-73-8
- Perindopril
Catalog No.:BCC4223
CAS No.:82834-16-0
- Pingpeimine A
Catalog No.:BCN8407
CAS No.:82841-67-6
- Pingpeimine B
Catalog No.:BCN8408
CAS No.:82851-52-3
- Echinacoside
Catalog No.:BCN4953
CAS No.:82854-37-3
- Cyclo(Tyr-Leu)
Catalog No.:BCN2432
CAS No.:82863-65-8
- 2,3-Dihydroisoginkgetin
Catalog No.:BCN4035
CAS No.:828923-27-9
A phase I study of danusertib (PHA-739358) in adult patients with accelerated or blastic phase chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant or intolerant to imatinib and/or other second generation c-ABL therapy.[Pubmed:25887498]
Haematologica. 2015 Jul;100(7):898-904.
Danusertib is a pan-aurora kinase inhibitor with potent activity against Abl kinase including the gatekeeper T315I mutant. A phase 1 dose escalation study of danusertib was conducted in patients with accelerated or blastic phase chronic myeloid leukemia or Philadelphia chromosome-positive acute lymphoblastic leukemia. Two dosing schedules were studied: schedule A, in which danusertib was given by 3-hour intravenous infusion daily for 7 consecutive days (days 1-7) in a 14-day cycle, and schedule B, in which the danusertib was given by 3-hour intravenous infusion daily for 14 consecutive days (days 1-14) in a 21-day cycle. A total of 37 patients were treated, 29 with schedule A and eight with schedule B. The recommended phase 2 dose for schedule A was 180 mg/m(2). Enrollment to schedule B was stopped early because of logistical problems with the frequency of infusions. Febrile neutropenia and mucositis were dose-limiting toxicities in schedule A. Four patients with T315I ABL kinase mutation, all treated with schedule A, responded. Danusertib has an acceptable toxicity profile and is active in patients with Bcr-Abl-associated advanced hematologic malignancies. This study was registered with the European Clinical Trails Data Base (EudraCT number 2007-004070-18).
Danusertib (formerly PHA-739358)--a novel combined pan-Aurora kinases and third generation Bcr-Abl tyrosine kinase inhibitor.[Pubmed:20072840]
Recent Results Cancer Res. 2010;184:199-214.
The Aurora kinases belong to a family of highly conserved serine/threonine protein kinases. They play an essential role as key mitotic regulators, controlling entry into mitosis, centrosome function, chromosome assembly, and segregation. As many other regulators of mitosis, Aurora kinases are frequently found to be aberrantly overexpressed in cancer cells. Therefore, these proteins have become an attractive target for the development of new anticancer therapies. In fact, several small-molecule inhibitors of Aurora kinases have already been developed and some of them have shown promising clinical efficacy in a number of human tumors in Phase I and II clinical trials. Among those, one of the most advanced clinical compound currently is Danusertib (formerly PHA-739358), which exhibits inhibitory activity against all known Aurora kinases as well as other cancer-relevant kinases such as the Bcr-Abl tyrosine kinase, including its multidrug-resistant T315I mutant. This mutation is responsible for up to 25% of all clinically observed resistances in CML patients undergoing Imatinib therapy. However, this particular mutation is predicted to play an even more important clinical role in the future, since in addition to Imatinib, it also confers resistance to second-generation Bcr-Abl inhibitors such as Nilotinib, Dasatinib, and Bosutinib. Therefore, combined Aurora and Bcr-Abl inhibition (the latter including high-grade resistance conferring mutations) with compounds such as Danusertib represents a promising new strategy for treatment of Bcr-Abl positive leukemias, especially those in second and third line of treatment.
Treatment of human pre-B acute lymphoblastic leukemia with the Aurora kinase inhibitor PHA-739358 (Danusertib).[Pubmed:22721004]
Mol Cancer. 2012 Jun 21;11:42.
BACKGROUND: Treatment of Philadelphia chromosome-positive acute lymphoblastic leukemias (Ph-positive ALL) with clinically approved inhibitors of the Bcr/Abl tyrosine kinase frequently results in the emergence of a leukemic clone carrying the T315I mutation in Bcr/Abl, which confers resistance to these drugs. PHA-739358, an Aurora kinase inhibitor, was reported to inhibit the Bcr/Abl T315I mutant in CML cells but no preclinical studies have examined this in detail in human ALL. RESULTS: We compared the sensitivity of human Bcr/Abl T315I, Bcr/Abl wild type and non-Bcr/Abl ALL cells to this drug. PHA-739358 inhibited proliferation and induced apoptosis independently of Bcr/Abl, the T315I mutation, or presence of the tumor suppressor p53, but the degree of effectiveness varied between different ALL samples. Since short-term treatment with a single dose of drug only transiently inhibited proliferation, we tested combination treatments of PHA-739358 with the farnesyltransferase inhibitor Lonafarnib, with vincristine and with dasatinib. All combinations reduced viability and cell numbers compared to treatment with a single drug. Clonogenic assays showed that 25 nM PHA-739358 significantly reduced the colony growth potential of Ph-positive ALL cells, and combined treatment with a second drug abrogated colony growth in this assay. PHA-739358 further effectively blocked Bcr/Abl tyrosine kinase activity and Aurora kinase B in vivo, and mice transplanted with human Bcr/Abl T315I ALL cells treated with a 3x 7-day cycle of PHA-739358 as mono-treatment had significantly longer survival. CONCLUSIONS: PHA-739358 represents an alternative drug for the treatment of both Ph-positive and negative ALL, although combined treatment with a second drug may be needed to eradicate the leukemic cells.
Targeting aurora kinases with danusertib (PHA-739358) inhibits growth of liver metastases from gastroenteropancreatic neuroendocrine tumors in an orthotopic xenograft model.[Pubmed:22753592]
Clin Cancer Res. 2012 Sep 1;18(17):4621-32.
PURPOSE: Aurora kinases play a crucial role in cell-cycle control. Uncontrolled expression of aurora kinases causes aneuploidy and tumor growth. As conservative treatment options for advanced gastroenteropancreatic neuroendocrine tumors (GEP-NET) are disappointing, aurora kinases may be an interesting target for novel therapeutic strategies. EXPERIMENTAL DESIGN: Human GEP-NETs were tested for aurora kinase expression. The efficacy of the new aurora kinase inhibitor danusertib was evaluated in two human GEP-NET cell lines (BON1 and QGP) in vitro and in vivo. RESULTS: The majority of ten insulinomas and all 33 nonfunctional pancreatic or midgut GEP-NETs expressed aurora A despite a mostly high degree of cell differentiation. Both human GEP-NET cell lines expressed aurora kinase A and B, and high Ser10 phosphorylation of histone H3 revealed increased aurora B activity. Remarkably, danusertib led to cell-cycle arrest and completely inhibited cell proliferation of the GEP-NET cells in vitro. Decreased phosphorylation of histone H3 indicated effective aurora B inhibition. In a subcutaneous murine xenograft model, danusertib significantly reduced tumor growth in vivo compared with controls or mice treated with streptozotocine/5-fluorouracil. As a consequence, decreased levels of tumor marker chromogranin A were found in mouse serum samples. In a newly developed orthotopic model for GEP-NET liver metastases by intrasplenic tumor cell transplantation, dynamic MRI proved significant growth inhibition of BON1- and QGP-derived liver metastases. CONCLUSIONS: These results show that danusertib may impose a new therapeutic strategy for aurora kinase expressing metastasized GEP-NETs.


