BGJ398FGFR inhibitor ,potent and selective CAS# 872511-34-7 |
- Regorafenib monohydrate
Catalog No.:BCC1884
CAS No.:1019206-88-2
- AZD4547
Catalog No.:BCC3711
CAS No.:1035270-39-3
- LY2874455
Catalog No.:BCC1723
CAS No.:1254473-64-7
- NVP-BGJ398 phosphate
Catalog No.:BCC1814
CAS No.:1310746-10-1
- Nintedanib (BIBF 1120)
Catalog No.:BCC3661
CAS No.:656247-17-5
- BIBF 1202
Catalog No.:BCC5298
CAS No.:894783-71-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 872511-34-7 | SDF | Download SDF |
| PubChem ID | 53235510 | Appearance | Powder |
| Formula | C26H31Cl2N7O3 | M.Wt | 560.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Infigratinib; BGJ-398 | ||
| Solubility | Soluble to 1 mg/mL warmed (1.78 mM) in DMSO | ||
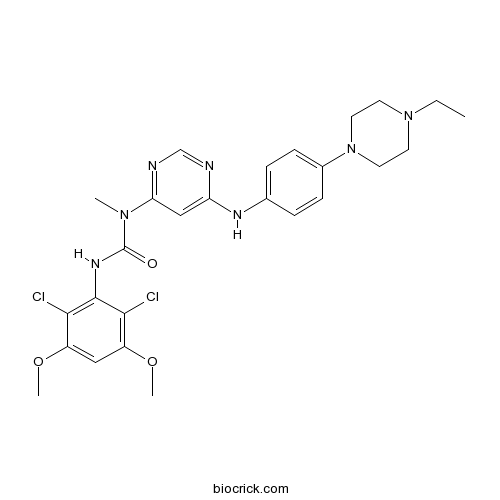
| Chemical Name | 3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-[6-[4-(4-ethylpiperazin-1-yl)anilino]pyrimidin-4-yl]-1-methylurea | ||
| SMILES | CCN1CCN(CC1)C2=CC=C(C=C2)NC3=CC(=NC=N3)N(C)C(=O)NC4=C(C(=CC(=C4Cl)OC)OC)Cl | ||
| Standard InChIKey | QADPYRIHXKWUSV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BGJ398 (NVP-BGJ398) is a potent and selective inhibitor of FGFR for FGFR1/2/3 with IC50 of 0.9 nM/1.4 nM/1 nM, >40-fold selective for FGFR versus FGFR4 and VEGFR2, and little activity to Abl, Fyn, Kit, Lck, Lyn and Yes. | ||||||
| Targets | FGFR1 | FGFR2 | FGFR3 | FGFR4 | |||
| IC50 | 0.9 nM | 1.4 nM | 1 nM | 60 nM | |||
| Cell experiment: [1] | |
| Cell lines | AN3CA, MFE296, MFE280, SNGM and HEC1A cells |
| Preparation method | The solubility of this compound in DMSO is <10 mm. general tips for obtaining a higher concentration: please warm the tube at 37 °c 10 minutes and> |
| Reacting condition | 0.5 μM, 72 hours |
| Applications | Exposure of AN3CA, MFE296, and MFE280 cells to the inhibitor led to a significant increase in the fraction of cells in G0–G1 arrest and to a significant increase in the fraction of cells undergoing apoptosis, when compared with untreated controls. In contrast, NVP-BGJ398 treatment did not alter the fractions of cells in G0–G1 arrest in the FGFR2 wild-type endometrial cancer cell lines SNGM or HEC1A in vitro. Moreover, NVP-BGJ398 treatment had no effect on apoptosis in the FGFR2 wild-type endometrial cancer cell line HEC1A. |
| Animal experiment: [1] | |
| Animal models | Nude mice bearing AN3CA, MFE296, SNGM or HEC1A xenografts |
| Dosage form | Oral administration, 30 or 50 mg/kg, daily |
| Application | NVP-BGJ398 significantly delayed the growth of FGFR2-mutated endometrial cancer xenograft tumors. In contrast, NVP-BGJ398 had no in vivo inhibitory effects in the long-term study using the FGFR2 wild-type endometrial cancer cell line SNGM, but surprisingly did show in vivo activity in HEC1A cells by delaying tumor growth in these cells. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Konecny G E, Kolarova T, O'Brien N A, et al. Activity of the fibroblast growth factor receptor inhibitors dovitinib (TKI258) and NVP-BGJ398 in human endometrial cancer cells. Molecular cancer therapeutics, 2013, 12(5): 632-642. | |

BGJ398 Dilution Calculator

BGJ398 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7842 mL | 8.9209 mL | 17.8418 mL | 35.6837 mL | 44.6046 mL |
| 5 mM | 0.3568 mL | 1.7842 mL | 3.5684 mL | 7.1367 mL | 8.9209 mL |
| 10 mM | 0.1784 mL | 0.8921 mL | 1.7842 mL | 3.5684 mL | 4.4605 mL |
| 50 mM | 0.0357 mL | 0.1784 mL | 0.3568 mL | 0.7137 mL | 0.8921 mL |
| 100 mM | 0.0178 mL | 0.0892 mL | 0.1784 mL | 0.3568 mL | 0.446 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
NVP-BGJ398, a selective FGFR inhibitor, is capable of inhibiting FGFR2 signaling, inducing cell-cycle arrest and increasing apoptosis in FGFR2-mutant endometrial cancer cells, which exhibits inhibition against cell growth both in vivo and in vitro.
Abstract
Genetic alterations in FGFR family members are potential predictors of NVP-BGJ398 sensitivity, including FGFR1 amplication in osteosarcoma and FGF19 copy number gain at the 11q13 amplicon.
Abstract
NVP-BGJ398 exhibited potent antitumor activity in RT112 bladder cancer xenografts models overexpressing wild-type FGFR3.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NVP-BGJ398 is a potent, selective, and orally bioavailable inhibitor of the FGFR tyrosine kinases. NVP-BGJ398 is a small molecular with the formula of C26H31Cl2N7O3 and Molecular Weight of 560. The fibroblast growth factor receptor 1 (FGFR1), FGFR2, FGFR3, and FGFR4, encompasses the receptors for 18 different FGF ligands. These ligand–receptor combinations regulate a broad spectrum of signaling during development and in normal growth control. BGJ398 inhibits the cell proliferation and induces apoptosis in cancer cells and suppresses tumor growth in xenograft model.
References:
1. Fibroblast Growth Factor Receptors as Novel Therapeutic Targets in SNF5-Deleted Malignant Rhabdoid Tumors. S Wöhrle, A Weiss, M Ito, A Kauffmann, M Murakami. PLOS ONE. 2013
2. Rescue screens with secreted proteins reveal compensatory potential of receptor tyrosine kinases in driving cancer growth. F Harbinski, VJ Craig, S Sanghavi, D Jeffery, L Liu. Cancer Discovery, 2012
- (+)-Noe's reagent
Catalog No.:BCC8377
CAS No.:87248-50-8
- Bijaponicaxanthone C
Catalog No.:BCN6884
CAS No.:872409-35-3
- NVP-QAV680
Catalog No.:BCC5508
CAS No.:872365-16-7
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
- K 114
Catalog No.:BCC5984
CAS No.:872201-12-2
- Dihydrotanshinone I
Catalog No.:BCN4417
CAS No.:87205-99-0
- WAY 207024 dihydrochloride
Catalog No.:BCC7802
CAS No.:872002-73-8
- 2-Deacetoxydecinnamoyltaxinine J
Catalog No.:BCN7218
CAS No.:87193-98-4
- Trametinib (GSK1120212)
Catalog No.:BCC1282
CAS No.:871700-17-3
- Schisanlactone A
Catalog No.:BCN3185
CAS No.:87164-31-6
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
- CTEP (RO4956371)
Catalog No.:BCC4599
CAS No.:871362-31-1
- Ro 3306
Catalog No.:BCC4007
CAS No.:872573-93-8
- 7-Epi-5-eudesmene-1beta,11-diol
Catalog No.:BCN7701
CAS No.:87261-77-6
- MEDICA 16
Catalog No.:BCC7956
CAS No.:87272-20-6
- Dabigatran etexilate mesylate
Catalog No.:BCC1511
CAS No.:872728-81-9
- 1,2,3,10-Tetramethoxy-9-(2-hydroxy-4,5-dimethoxybenzyloxy)oxoaporphine
Catalog No.:BCN8120
CAS No.:872729-33-4
- 3-Methoxyoxohernandaline
Catalog No.:BCN8107
CAS No.:872729-34-5
- 6-Aminouracil
Catalog No.:BCC8768
CAS No.:873-83-6
- 3,29-Dibenzoyl karounitriol
Catalog No.:BCN2717
CAS No.:873001-54-8
- Ivacaftor (VX-770)
Catalog No.:BCC2478
CAS No.:873054-44-5
- Ganoderone A
Catalog No.:BCN2448
CAS No.:873061-79-1
- IKK-16 (IKK Inhibitor VII)
Catalog No.:BCC4555
CAS No.:873225-46-8
- AIM-100
Catalog No.:BCC1333
CAS No.:873305-35-2
Evaluation of BGJ398, a Fibroblast Growth Factor Receptor 1-3 Kinase Inhibitor, in Patients With Advanced Solid Tumors Harboring Genetic Alterations in Fibroblast Growth Factor Receptors: Results of a Global Phase I, Dose-Escalation and Dose-Expansion Study.[Pubmed:27870574]
J Clin Oncol. 2017 Jan 10;35(2):157-165.
Purpose This two-part, first-in-human study was initiated in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors (FGFRs) to determine the maximum tolerated dose (MTD), the recommended phase II dose (RP2D), and the schedule, safety, pharmacokinetics, pharmacodynamics, and antitumor activity of oral BGJ398, a selective FGFR1-3 tyrosine kinase inhibitor. Patients and Methods Adult patients were treated with escalating dosages of BGJ398 5 to 150 mg once daily or 50 mg twice daily continuously in 28-day cycles. During expansion at the MTD, patients with FGFR1-amplified squamous cell non-small-cell lung cancer (sqNSCLC; arm 1) or other solid tumors with FGFR genetic alterations (mutations/amplifications/fusions) received BGJ398 daily on a continuous schedule (arm 2), or on a 3-weeks-on/1-week-off schedule (arm 3). Results Data in 132 patients from the escalation and expansion arms are reported (May 15, 2015, cutoff). The MTD, 125 mg daily, was determined on the basis of dose-limiting toxicities in four patients (100 mg, grade 3 aminotransferase elevations [n = 1]; 125 mg, hyperphosphatemia [n = 1]; 150 mg, grade 1 corneal toxicity [n = 1] and grade 3 aminotransferase elevations [n = 1]). Common adverse events in patients treated at the MTD (n = 57) included hyperphosphatemia (82.5%), constipation (50.9%), decreased appetite (45.6%), and stomatitis (45.6%). A similar safety profile was observed using the 3-weeks-on/1-week-off schedule (RP2D). However, adverse event-related dose adjustments/interruptions were less frequent with the 3-weeks-on/1-week-off (50.0%) versus the continuous (73.7%) schedule. Antitumor activity (seven partial responses [six confirmed]) was demonstrated with BGJ398 doses >/= 100 mg in patients with FGFR1-amplified sqNSCLC and FGFR3-mutant bladder/urothelial cancer. Conclusion BGJ398 at the MTD/RP2D had a tolerable and manageable safety profile and showed antitumor activity in several tumor types, including FGFR1-amplified sqNSCLC and FGFR3-mutant bladder/urothelial cancers.
Akt Activation Mediates Acquired Resistance to Fibroblast Growth Factor Receptor Inhibitor BGJ398.[Pubmed:28255027]
Mol Cancer Ther. 2017 Apr;16(4):614-624.
Activation of FGFR signaling through mutations, amplifications, or fusions involving FGFR1, 2, 3, or 4 is seen in multiple tumors, including lung, bladder, and cholangiocarcinoma. Currently, several clinical trials are evaluating the role of novel FGFR inhibitors in solid tumors. As we move forward with FGFR inhibitors clinically, we anticipate the emergence of resistance with treatment. Consequently, we sought to study the mechanism(s) of acquired resistance to FGFR inhibitors using annotated cancer cell lines. We identified cancer cell lines that have activating mutations in FGFR1, 2, or 3 and treated them chronically with the selective FGFR inhibitor, BGJ398. We observed resistance to chronic BGJ398 exposure in DMS114 (small-cell lung cancer, FGFR1 amplification) and RT112 (urothelial carcinoma, FGFR3 fusion/amplification) cell lines based on viability assays. Reverse-phase protein array (RPPA) analysis showed increased phosphorylation of Akt (T308 and S473) and its downstream target GSK3 (S9 and S21) in both the resistant cell lines when compared with matching controls. Results of RPPA were confirmed using immunoblots. Consequently, the addition of an Akt inhibitor (GSK2141795) or siRNA was able to restore sensitivity to BGJ398 in resistant cell lines. These data suggest a role for Akt pathway in mediating acquired resistance to FGFR inhibition. Mol Cancer Ther; 16(4); 614-24. (c)2017 AACR.
Selective FGFR inhibitor BGJ398 inhibits phosphorylation of AKT and STAT3 and induces cytotoxicity in sphere-cultured ovarian cancer cells.[Pubmed:28350116]
Int J Oncol. 2017 Mar 15.
Epithelial ovarian cancer is the most aggressive and lethal among the gynecological malignancies, which is often found disseminated to peritoneal cavity at the time of diagnosis. There is accumulating evidence on the existence of genetic alteration and amplification of fibroblast growth factor receptor (FGFR) in various cancers. Also the aberrated FGFR/FGF signaling has been implicated in cancer development and tumor microenvironment. However, the antitumor activity of BGJ398, a selective inhibitor of FGFR 1/2/3 against ovarian cancer still remains unknown. The aim of the present study is to evaluate the antitumoral activity of BGJ398 on ovarian cancer cell line SKOV3ip1 using 3-dimensional (3D) sphere culture system which has been accepted as a better mimic in vivo microenvironment than conventional 2-dimensional (2D) monolayer culture system. We examined the differential expression features of key signaling molecules which have a role in cell survival and proliferation between sphere-cultured SKOV3ip1 cells and monolayer-cultured SKOV3ip1 cells. The phosphorylation of AKT and signal transducer and activator of transcription 3 (STAT3) known as survival signaling molecules were upregulated in sphere-cultured SKOV3ip1 cells compared to in monolayer-cultured SKOV3ip1 cells. Next, we evaluated the antitumor activity of BGJ398 in monolayer-cultured SKOV3ip1 cells or sphere-cultured SKOV3ip1 cells. Treatment of BGJ398 did not affect the SKOV3ip1 cell viability in monolayer culture system, but, the cell viability of sphere-cultured SKOV3ip1 cells was markedly reduced by BGJ398. The phosphorylation of AKT and STAT3 was downregulated by BGJ398 in sphere-cultured SKOV3ip1 cells, but not in monolayer cultured-SKOV3ip1 cells. Moreover, combination treatment with BGJ398 and paclitaxel in sphere-cultured SKOV3ip1 showed synergistic inhibitory effect on cell viability. Collectively, our report reveals the BGJ398 is a potent antitumor agent against ovarian cancer and FGFR is a promising therapeutic target to anticancer therapy considering ovarian cancer metastatic microenvironment.


