AIM-100Ack1 inhibitor CAS# 873305-35-2 |
- AVL-292 benzenesulfonate
Catalog No.:BCC1386
CAS No.:1360053-81-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 873305-35-2 | SDF | Download SDF |
| PubChem ID | 11501591 | Appearance | Powder |
| Formula | C23H21N3O2 | M.Wt | 371.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (134.61 mM; Need ultrasonic) | ||
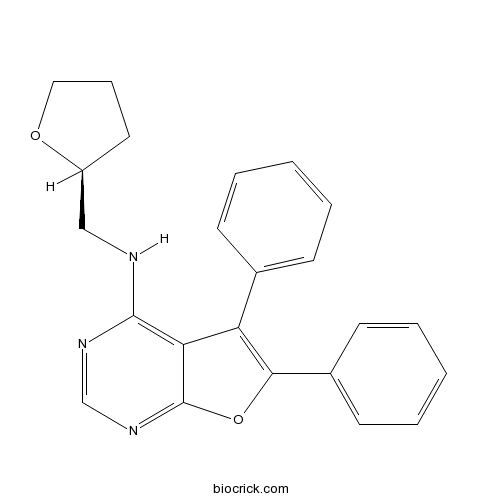
| Chemical Name | N-[[(2S)-oxolan-2-yl]methyl]-5,6-diphenylfuro[2,3-d]pyrimidin-4-amine | ||
| SMILES | C1CC(OC1)CNC2=C3C(=C(OC3=NC=N2)C4=CC=CC=C4)C5=CC=CC=C5 | ||
| Standard InChIKey | XNFHHOXCDUAYSR-SFHVURJKSA-N | ||
| Standard InChI | InChI=1S/C23H21N3O2/c1-3-8-16(9-4-1)19-20-22(24-14-18-12-7-13-27-18)25-15-26-23(20)28-21(19)17-10-5-2-6-11-17/h1-6,8-11,15,18H,7,12-14H2,(H,24,25,26)/t18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AIM-100 is a small molecule inhibitor of Ack1 with an IC50 of 24 nM.
IC50 value: 24 nM [3]
Target: Ack1
Ack1 inhibitor AIM-100 not only inhibited Ack1 activation but also suppressed AKT tyrosine phosphorylation, leading to cell cycle arrest in the G1 phase [1].The Ack1 inhibitor AIM-100 not only inhibited Ack1 activity but also was able to suppress AR Tyr(267) phosphorylation and its recruitment to the ATM enhancer. Notably, AIM-100 suppressed Ack1 mediated ATM expression and mitigated the growth of radioresistant CRPC tumors [2]. AIM-100, not only inhibited Ack1 activation but also able to suppress pTyr267-AR phosphorylation, binding of AR to PSA, NKX3.1, and TMPRSS2 promoters, and inhibit AR transcription activity [3]. References: | |||||

AIM-100 Dilution Calculator

AIM-100 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6923 mL | 13.4615 mL | 26.923 mL | 53.8459 mL | 67.3074 mL |
| 5 mM | 0.5385 mL | 2.6923 mL | 5.3846 mL | 10.7692 mL | 13.4615 mL |
| 10 mM | 0.2692 mL | 1.3461 mL | 2.6923 mL | 5.3846 mL | 6.7307 mL |
| 50 mM | 0.0538 mL | 0.2692 mL | 0.5385 mL | 1.0769 mL | 1.3461 mL |
| 100 mM | 0.0269 mL | 0.1346 mL | 0.2692 mL | 0.5385 mL | 0.6731 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AIM-100 is a small inhibitor of Ack1 tyrosine kinase with IC50 value of 24 nM [1].
AIM-100 mimicked ATP and inhibited the activity of Ack1 significantly and specifically. It showed no inhibition activity for the other 30 kinases including PI3-kinase and AKT, and showed a five-fold higher IC50 value for Lck. In MEF cells treated with EGF, AIM-100 caused a remarkable decrease of the activation of Ack1. In the human prostate cancer cells LNCaP and LAPC4, AIM-100 treatment resulted in an increase of G0/G1 cell phase and subsequent cell growth suppression. These effects of AIM-100 also exerted in the pancreatic cancer cells. AIM-100 induced apoptosis in Panc-1 cells at concentration of 10 μM. Moreover, AIM-100 inhibited cell growth with GI50 values of 7 to 8 μM in CD-18, Panc-1, OV90, MCF-7 and MDA-MB-468 cancer cells [1, 2].
References:
1. Mahajan K, Challa S, Coppola D, et al. Effect of Ack1 tyrosine kinase inhibitor on ligand-independent androgen receptor activity. The Prostate, 2010, 70(12): 1274-1285.
2. Mahajan K, Coppola D, Chen Y, et al. Ack1 tyrosine kinase activation correlates with pancreatic cancer progression. The American journal of pathology, 2012, 180(4): 1386-1393.
- IKK-16 (IKK Inhibitor VII)
Catalog No.:BCC4555
CAS No.:873225-46-8
- Ganoderone A
Catalog No.:BCN2448
CAS No.:873061-79-1
- Ivacaftor (VX-770)
Catalog No.:BCC2478
CAS No.:873054-44-5
- 3,29-Dibenzoyl karounitriol
Catalog No.:BCN2717
CAS No.:873001-54-8
- 6-Aminouracil
Catalog No.:BCC8768
CAS No.:873-83-6
- 3-Methoxyoxohernandaline
Catalog No.:BCN8107
CAS No.:872729-34-5
- 1,2,3,10-Tetramethoxy-9-(2-hydroxy-4,5-dimethoxybenzyloxy)oxoaporphine
Catalog No.:BCN8120
CAS No.:872729-33-4
- Dabigatran etexilate mesylate
Catalog No.:BCC1511
CAS No.:872728-81-9
- MEDICA 16
Catalog No.:BCC7956
CAS No.:87272-20-6
- 7-Epi-5-eudesmene-1beta,11-diol
Catalog No.:BCN7701
CAS No.:87261-77-6
- Ro 3306
Catalog No.:BCC4007
CAS No.:872573-93-8
- BGJ398
Catalog No.:BCC1278
CAS No.:872511-34-7
- Ramipril
Catalog No.:BCC5012
CAS No.:87333-19-5
- 1,7-Dihydroxy-4-methoxyxanthone
Catalog No.:BCN7602
CAS No.:87339-76-2
- TC-P 262
Catalog No.:BCC6155
CAS No.:873398-67-5
- Fortuneine
Catalog No.:BCN6401
CAS No.:87340-25-8
- Triangularine
Catalog No.:BCN2051
CAS No.:87340-27-0
- PU-H71
Catalog No.:BCC1872
CAS No.:873436-91-0
- Lupeolic acid
Catalog No.:BCN6520
CAS No.:87355-32-6
- GDC-0152
Catalog No.:BCC2252
CAS No.:873652-48-3
- Demethylcarolignan E
Catalog No.:BCN7018
CAS No.:873694-46-3
- Omecamtiv mecarbil
Catalog No.:BCC3710
CAS No.:873697-71-3
- PLX647
Catalog No.:BCC6370
CAS No.:873786-09-5
- TATU
Catalog No.:BCC2822
CAS No.:873798-09-5
Ack1 tyrosine kinase activation correlates with pancreatic cancer progression.[Pubmed:22322295]
Am J Pathol. 2012 Apr;180(4):1386-93.
Pancreatic cancer is a significant cause of cancer mortality worldwide as the disease has advanced significantly in patients before symptoms are evident. The signal transduction pathways that promote this rapid progression are not well understood. Ack1 or TNK2, an ubiquitously expressed oncogenic non-receptor tyrosine kinase, integrates signals from ligand-activated receptor tyrosine kinases to modulate intracellular signaling cascades. In the present study, we investigated the Ack1 activation profile in a pancreatic cancer tumor microarray, and observed that expression levels of activated Ack1 and pTyr284-Ack1 positively correlated with the severity of disease progression and inversely correlated with the survival of patients with pancreatic cancer. To explore the mechanisms by which Ack1 promotes tumor progression, we investigated the role of AKT/PKB, an oncogene and Ack1-interacting protein. Ack1 activates AKT directly in pancreatic and other cancer cell lines by phosphorylating AKT at Tyr176 to promote cell survival. In addition, the Ack1 inhibitor AIM-100 not only inhibited Ack1 activation but also suppressed AKT tyrosine phosphorylation, leading to cell cycle arrest in the G1 phase. This effect resulted in a significant decrease in the proliferation of pancreatic cancer cells and induction of apoptosis. Collectively, our data indicate that activated Ack1 could be a prognostic marker for ascertaining early or advanced pancreatic cancer. Thus, Ack1 inhibitors hold promise for therapeutic intervention to inhibit pancreatic tumor growth.
ACK1 tyrosine kinase interacts with histone demethylase KDM3A to regulate the mammary tumor oncogene HOXA1.[Pubmed:25148682]
J Biol Chem. 2014 Oct 10;289(41):28179-91.
Hormone therapy with the selective estrogen-receptor modulator tamoxifen provides a temporary relief for patients with estrogen receptor alpha (ER)-positive breast cancers. However, a subset of patients exhibiting overexpression of the HER2 receptor tyrosine kinase displays intrinsic resistance to tamoxifen therapy. Therefore, elucidating the mechanisms promoting the estrogen (E2)-independent ER-regulated gene transcription in tamoxifen-resistant breast tumors is essential to identify new therapeutic avenues to overcome drug resistance and ameliorate poor prognosis. The non-receptor tyrosine kinase, ACK1 (also known as TNK2), has emerged as a major integrator of signaling from various receptor tyrosine kinases including HER2. We have uncovered that heregulin-mediated ACK1 activation promoted ER activity in the presence of tamoxifen, which was significantly down-regulated upon ACK1 knockdown or inhibition of ACK1 by small molecule inhibitors, AIM-100 or Dasatinib. We report that ACK1 phosphorylates the ER co-activator, KDM3A, a H3K9 demethylase, at an evolutionary conserved tyrosine 1114 site in a heregulin-dependent manner, even in the presence of tamoxifen. Consistent with this finding, ACK1 activation resulted in a significant decrease in the deposition of dimethyl H3K9 epigenetic marks. Conversely, inhibition of ACK1 by AIM-100 or Dasatinib restored dimethyl H3K9 methylation marks and caused transcriptional suppression of the ER-regulated gene HOXA1. Thus, by its ability to regulate the epigenetic activity of an ER co-activator KDM3A, ACK1 modulates HOXA1 expression in the absence of E2, conferring tamoxifen resistance. These data reveal a novel therapeutic option, suppression of ACK1 signaling by AIM-100 or Dasatinib, to mitigate HOXA1 up-regulation in breast cancer patients displaying tamoxifen resistance.
Effect of Ack1 tyrosine kinase inhibitor on ligand-independent androgen receptor activity.[Pubmed:20623637]
Prostate. 2010 Sep 1;70(12):1274-85.
BACKGROUND: Androgen receptor (AR) plays a critical role in the progression of both androgen-dependent and androgen-independent prostate cancer (AIPC). Ligand-independent activation of AR in AIPC or castration resistant prostate cancer (CRPC) is often associated with poor prognosis. Recently, tyrosine kinase Ack1 has been shown to regulate AR activity by phosphorylating it at tyrosine 267 and this event was shown to be critical for AIPC growth. However, whether a small molecule inhibitor that can mitigate Ack1 activation is sufficient to abrogate AR activity on AR regulated promoters in androgen-depleted environment is not known. METHODS: We have generated two key resources, antibodies that specifically recognize pTyr267-AR and synthesized a small molecule inhibitor of Ack1, 4-amino-5,6-biaryl-furo[2,3-d]pyrimidine (named here as AIM-100) to test whether AIM-100 modulates ligand-independent AR activity and inhibits prostate cell growth. RESULTS: Prostate tissue microarray analysis indicates that Ack1 Tyr284 phosphorylation correlates positively with disease progression and negatively with the survival of prostate cancer patients. Interestingly, neither pTyr267-AR expression nor its transcriptional activation was affected by anti-androgens in activated Ack1 expressing or EGF stimulated prostate cells. However, the Ack1 inhibitor, AIM-100, not only inhibited Ack1 activation but also able to suppress pTyr267-AR phosphorylation, binding of AR to PSA, NKX3.1, and TMPRSS2 promoters, and inhibit AR transcription activity. CONCLUSION: Ack1 Tyr284 phosphorylation is prognostic of progression of prostate cancer and inhibitors of Ack1 activity could be novel therapeutic agents to treat AIPC.
Ack1-mediated androgen receptor phosphorylation modulates radiation resistance in castration-resistant prostate cancer.[Pubmed:22566699]
J Biol Chem. 2012 Jun 22;287(26):22112-22.
Androgen deprivation therapy has been the standard of care in prostate cancer due to its effectiveness in initial stages. However, the disease recurs, and this recurrent cancer is referred to as castration-resistant prostate cancer (CRPC). Radiotherapy is the treatment of choice; however, in addition to androgen independence, CRPC is often resistant to radiotherapy, making radioresistant CRPC an incurable disease. The molecular mechanisms by which CRPC cells acquire radioresistance are unclear. Androgen receptor (AR)-tyrosine 267 phosphorylation by Ack1 tyrosine kinase (also known as TNK2) has emerged as an important mechanism of CRPC growth. Here, we demonstrate that pTyr(267)-AR is recruited to the ATM (ataxia telangiectasia mutated) enhancer in an Ack1-dependent manner to up-regulate ATM expression. Mice engineered to express activated Ack1 exhibited a significant increase in pTyr(267)-AR and ATM levels. Furthermore, primary human CRPCs with up-regulated activated Ack1 and pTyr(267)-AR also exhibited significant increase in ATM expression. The Ack1 inhibitor AIM-100 not only inhibited Ack1 activity but also was able to suppress AR Tyr(267) phosphorylation and its recruitment to the ATM enhancer. Notably, AIM-100 suppressed Ack1 mediated ATM expression and mitigated the growth of radioresistant CRPC tumors. Thus, our study uncovers a previously unknown mechanism of radioresistance in CRPC, which can be therapeutically reversed by a new synergistic approach that includes radiotherapy along with the suppression of Ack1/AR/ATM signaling by the Ack1 inhibitor, AIM-100.
Activated Cdc42-associated kinase 1 (ACK1) binds the sterile alpha motif (SAM) domain of the adaptor SLP-76 and phosphorylates proximal tyrosines.[Pubmed:28188290]
J Biol Chem. 2017 Apr 14;292(15):6281-6290.
The adaptor protein Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76) plays a crucial role in T cell activation by linking antigen receptor (T cell receptor, TCR) signals to downstream pathways. At its N terminus, SLP-76 has three key tyrosines (Tyr-113, Tyr-128, and Tyr-145, "3Y") as well as a sterile alpha motif (SAM) domain whose function is unclear. We showed previously that the SAM domain has two binding regions that mediate dimer and oligomer formation. In this study, we have identified SAM domain-carrying non-receptor tyrosine kinase, activated Cdc42-associated tyrosine kinase 1 (ACK1; also known as Tnk2, tyrosine kinase non-receptor 2) as a novel binding partner of SLP-76. Co-precipitation, laser-scanning confocal microscopy, and in situ proximity analysis confirmed the binding of ACK1 to SLP-76. Further, the interaction was induced in response to the anti-TCR ligation and abrogated by the deletion of SLP-76 SAM domain (DeltaSAM) or mutation of Tyr-113, Tyr-128, and Tyr-145 to phenylalanine (3Y3F). ACK1 induced phosphorylation of the SLP-76 N-terminal tyrosines (3Y) dependent on the SAM domain. Further, ACK1 promoted calcium flux and NFAT-AP1 promoter activity and decreased the motility of murine CD4(+) primary T cells on ICAM-1-coated plates, an event reversed by a small molecule inhibitor of ACK1 (AIM-100). These findings identify ACK1 as a novel SLP-76-associated protein-tyrosine kinase that modulates early activation events in T cells.


