MEDICA 16ATP citrate lyase inhibitor; also inhibits FFA1 CAS# 87272-20-6 |
- Salmefamol
Catalog No.:BCC1919
CAS No.:18910-65-1
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- (R,R)-Formoterol
Catalog No.:BCC1293
CAS No.:67346-49-0
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 87272-20-6 | SDF | Download SDF |
| PubChem ID | 121871 | Appearance | Powder |
| Formula | C20H38O4 | M.Wt | 342.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
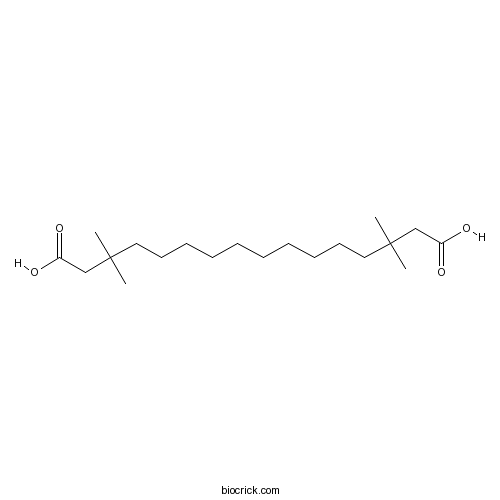
| Chemical Name | 3,3,14,14-tetramethylhexadecanedioic acid | ||
| SMILES | CC(C)(CCCCCCCCCCC(C)(C)CC(=O)O)CC(=O)O | ||
| Standard InChIKey | HYSMCRNFENOHJH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H38O4/c1-19(2,15-17(21)22)13-11-9-7-5-6-8-10-12-14-20(3,4)16-18(23)24/h5-16H2,1-4H3,(H,21,22)(H,23,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Free fatty acid 1 (FFA1/GPR40) receptor agonist; exhibits selectivity for FFA1 (GPR40) over GPR120. Also inhibits ATP citrate lyase; reduces intracellular triacylglycerol levels in muscle, inhibits lipogenesis and increases insulin sensitivity in rodents in vivo. |

MEDICA 16 Dilution Calculator

MEDICA 16 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9196 mL | 14.5981 mL | 29.1962 mL | 58.3925 mL | 72.9906 mL |
| 5 mM | 0.5839 mL | 2.9196 mL | 5.8392 mL | 11.6785 mL | 14.5981 mL |
| 10 mM | 0.292 mL | 1.4598 mL | 2.9196 mL | 5.8392 mL | 7.2991 mL |
| 50 mM | 0.0584 mL | 0.292 mL | 0.5839 mL | 1.1678 mL | 1.4598 mL |
| 100 mM | 0.0292 mL | 0.146 mL | 0.292 mL | 0.5839 mL | 0.7299 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7-Epi-5-eudesmene-1beta,11-diol
Catalog No.:BCN7701
CAS No.:87261-77-6
- Ro 3306
Catalog No.:BCC4007
CAS No.:872573-93-8
- BGJ398
Catalog No.:BCC1278
CAS No.:872511-34-7
- (+)-Noe's reagent
Catalog No.:BCC8377
CAS No.:87248-50-8
- Bijaponicaxanthone C
Catalog No.:BCN6884
CAS No.:872409-35-3
- NVP-QAV680
Catalog No.:BCC5508
CAS No.:872365-16-7
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
- K 114
Catalog No.:BCC5984
CAS No.:872201-12-2
- Dihydrotanshinone I
Catalog No.:BCN4417
CAS No.:87205-99-0
- WAY 207024 dihydrochloride
Catalog No.:BCC7802
CAS No.:872002-73-8
- 2-Deacetoxydecinnamoyltaxinine J
Catalog No.:BCN7218
CAS No.:87193-98-4
- Trametinib (GSK1120212)
Catalog No.:BCC1282
CAS No.:871700-17-3
- Dabigatran etexilate mesylate
Catalog No.:BCC1511
CAS No.:872728-81-9
- 1,2,3,10-Tetramethoxy-9-(2-hydroxy-4,5-dimethoxybenzyloxy)oxoaporphine
Catalog No.:BCN8120
CAS No.:872729-33-4
- 3-Methoxyoxohernandaline
Catalog No.:BCN8107
CAS No.:872729-34-5
- 6-Aminouracil
Catalog No.:BCC8768
CAS No.:873-83-6
- 3,29-Dibenzoyl karounitriol
Catalog No.:BCN2717
CAS No.:873001-54-8
- Ivacaftor (VX-770)
Catalog No.:BCC2478
CAS No.:873054-44-5
- Ganoderone A
Catalog No.:BCN2448
CAS No.:873061-79-1
- IKK-16 (IKK Inhibitor VII)
Catalog No.:BCC4555
CAS No.:873225-46-8
- AIM-100
Catalog No.:BCC1333
CAS No.:873305-35-2
- Ramipril
Catalog No.:BCC5012
CAS No.:87333-19-5
- 1,7-Dihydroxy-4-methoxyxanthone
Catalog No.:BCN7602
CAS No.:87339-76-2
- TC-P 262
Catalog No.:BCC6155
CAS No.:873398-67-5
MEDICA 16 inhibits hepatic acetyl-CoA carboxylase and reduces plasma triacylglycerol levels in insulin-resistant JCR: LA-cp rats.[Pubmed:11978655]
Diabetes. 2002 May;51(5):1548-55.
Intracellular triacylglycerol (TG) content of liver and skeletal muscle contributes to insulin resistance, and a significant correlation exists between TG content and the development of insulin resistance. Because acetyl-CoA carboxylase (ACC) is the rate-limiting enzyme for liver fatty acid biosynthesis and a key regulator of muscle fatty acid oxidation, we examined whether ACC plays a role in the accumulation of intracellular TG. We also determined the potential role of 5'-AMP-activated protein kinase (AMPK) in this process, since it can phosphorylate and inhibit ACC activity in both liver and muscle. TG content, ACC, and AMPK were examined in the liver and skeletal muscle of insulin-resistant JCR:LA-cp rats during the time frame when insulin resistance develops. At 12 weeks of age, there was a threefold elevation in liver TG content and a sevenfold elevation in skeletal muscle TG content. Hepatic ACC activity was significantly elevated in 12-week-old JCR:LA-cp rats compared with lean age-matched controls (8.75 +/- 0.53 vs. 3.30 +/- 0.18 nmol. min(-1). mg(-1), respectively), even though AMPK activity was also increased. The observed increase in hepatic ACC activity was accompanied by a 300% increase in ACC protein expression. There were no significant differences in ACC activity, ACC protein expression, or AMPK activity in the skeletal muscle of the 12-week JCR:LA-cp rats. Treatment of 12-week JCR:LA-cp rats with MEDICA 16 (an ATP-citrate lyase inhibitor) resulted in a decrease in hepatic ACC and AMPK activities, but had no effect on skeletal muscle ACC and AMPK. Our data suggest that alterations in ACC or AMPK activity in muscle do not contribute to the development of insulin resistance. However, increased liver ACC activity in the JCR:LA-cp rat appears to contribute to the development of lipid abnormalities, although this increase does not appear to occur secondary to a decrease in AMPK activity.
Peroxisome proliferators as adjuvants for the reverse-electron-transport therapy of obesity: an explanation for the large increase in metabolic rate of MEDICA 16-treated rats.[Pubmed:10608261]
Med Hypotheses. 1999 Oct;53(4):272-6.
The efficacy of reverse-electron-transport therapy of obesity should be promoted by agents which up-regulate hepatocyte enzymes that are potentially rate-limiting for mitochondrial fatty acid oxidation and electron shuttles. Peroxisome proliferator drugs, including the fibrates used to treat hyperlipidemia, may be useful in this regard, as they induce malic enzyme, the mitochondrial glycerol-3-phosphate dehydrogenase, and carnitine palmitoyl transferase I in rodent hepatocytes. An agent of this class, MEDICA 16, has the additional property of potently inhibiting both citrate lyase and acetyl-CoA carboxylase. As a result, methyl-substituted diacarboxylic acids (MEDICA) 16 can be expected to disinhibit hepatic fatty acid oxidation while up-regulating electron shuttle mechanisms, and thus should stimulate reverse electron transport. This may explain the remarkable 40% increase in basal metabolic rate observed in normal rats ingesting MEDICA 16--an effect not associated with any compensatory increase in food intake. Relative to controls, the MEDICA 16-treated rats achieved a 50% reduction in body fat and a modest increase in lean mass, such that weight and growth were not changed. In other rodent strains, MEDICA 16 has prevented obesity diabetes and atherogenesis. However, whether MEDICA 16 and other peroxisome proliferator drugs will have clinical utility in reverse-electron-transport therapy may hinge on their ability to induce key enzymes in human hepatocytes; cell culture studies to evaluate this are required.
Development of insulin resistance in the JCR:LA-cp rat: role of triacylglycerols and effects of MEDICA 16.[Pubmed:9588449]
Diabetes. 1998 May;47(5):770-8.
The JCR:LA-cp rat develops an extreme obese/insulin-resistant syndrome such that by 12 weeks of age, there is no longer any insulin-mediated glucose turnover. At 4 weeks of age, obese and lean rats have essentially identical basal and insulin-mediated glucose uptake in skeletal muscle. By 8 weeks of age, however, the obese rats no longer exhibit such intake. Plasma insulin concentrations in the normal fed state show only small increases up to 4 weeks, with a rapid rise to a marked hyperinsulinemia thereafter, with an age at half-development of 5.5 weeks. Plasma triacylglycerol concentrations in fed obese rats are elevated at 3 weeks and rise rapidly thereafter. The triacylglycerol content of skeletal muscle is significantly elevated in the obese rats at 4 weeks of age. Histological examination of Oil Red O-stained muscle tissue and transmission electron microscopy shows the presence of intracellular lipid droplets. Treatment with the potent triacylglycerol-lowering agent MEDICA 16 (beta,beta'-tetramethylhexadecanedioic acid) from 6 weeks of age reduces plasma lipids markedly, but it reduces body weight and insulin resistance only modestly. In contrast, treatment with MEDICA 16 from the time of weaning at 3 weeks of age results in the normalization of food intake and body weight to over 8 weeks of age. The development of hyperinsulinemia is also delayed until 8.5 weeks of age, and insulin levels remain strongly reduced. Plasma triacylglycerol concentrations remain at the same level as in lean rats, and neither an elevated muscle triacylglycerol content nor intracellular lipid droplets are found at 4 weeks of age. The results indicate that insulin resistance develops in the young animals and is not directly due to a genetically determined defect in insulin metabolism. The mechanism of induction instead appears to be related to an exaggerated triacylglycerol metabolism.
Adipose tissue sensitization to insulin induced by troglitazone and MEDICA 16 in obese Zucker rats in vivo.[Pubmed:12488241]
Am J Physiol Endocrinol Metab. 2003 Apr;284(4):E795-803.
The putative role played by insulin sensitizers in modulating adipose tissue lipolysis in the fasting state was evaluated in obese conscious Zucker rats treated with troglitazone or beta,beta'-tetramethylhexadecanedioic acid (MEDICA 16) and compared with nontreated lean and obese animals. The rates of appearance (R(a)) of glycerol and free fatty acid (FFA), primary intra-adipose reesterification, and secondary reuptake of plasma FFA in adipose fat were measured using constant infusion of stable isotope-labeled [(2)H(5)]glycerol, [2,2-(2)H(2)]palmitate, and radioactive [(3)H]palmitate. The overall lipolytic flux (R(a) glycerol) was increased 1.7- and 1.4-fold in obese animals treated with troglitazone or MEDICA 16, respectively, resulting in increased FFA export (R(a) FFA) in the troglitazone-treated rats. Primary intra-adipose reesterification of lipolysis-derived fatty acids was enhanced twofold by insulin sensitizers, whereas reesterification of plasma fatty acids was unaffected by either treatment. Despite the unchanged R(a) FFA in MEDICA 16 or the increased R(a) FFA induced by troglitazone, very low density lipoprotein production rates were robustly curtailed. Total adipose tissue reesterification, used as an estimate of glucose conversion to glyceride-glycerol, was increased 1.9-fold by treatment with the insulin sensitizers. Our results indicate that, in the fasting state, insulin sensitizers induce, in vivo, a significant activation rather than suppression of adipose tissue lipolysis together with stimulation of glucose conversion to glyceride-glycerol.
Novel selective ligands for free fatty acid receptors GPR120 and GPR40.[Pubmed:19471906]
Naunyn Schmiedebergs Arch Pharmacol. 2009 Sep;380(3):247-55.
GPR120 and GPR40 are G-protein-coupled receptors whose endogenous ligands are medium- and long-chain free fatty acids, and they are thought to play an important physiological role in insulin release. Despite recent progress in understanding their roles, much still remains unclear about their pharmacology, and few specific ligands for GPR120 and GPR40 besides medium- to long-chain fatty acids have been reported so far. To identify new selective ligands for these receptors, more than 80 natural compounds were screened, together with a reference compound MEDICA16, which is known to activate GPR40, by monitoring the extracellular regulated kinase (ERK) and [Ca(2+)](i) responses in inducible and stable expression cell lines for GPR40 and GPR120, respectively. MEDICA16 selectively activated [Ca(2+)](i) response in GPR40-expressing cells but not in GPR120-expressing cells. Among the natural compounds tested, grifolin derivatives, grifolic acid and grifolic acid methyl ether, promoted ERK and [Ca(2+)](i) responses in GPR120-expressing cells, but not in GPR40-expressing cells, and inhibited the alpha-linolenic acid (LA)-induced ERK and [Ca(2+)](i) responses in GPR120-expressing cells. Interestingly, in accordance with the pharmacological profiles of these compounds, similar profiles of glucagon-like peptide-1 secretion were seen for mouse enteroendocrine cell line, STC-1 cells, which express GPR120 endogenously. Taken together, these studies identified a selective GPR40 agonist and several GPR120 partial agonists. These compounds would be useful probes to further investigate the physiological and pharmacological functions of GPR40 and GPR120.


