TATUCAS# 873798-09-5 |
- Quercetin
Catalog No.:BCN6049
CAS No.:117-39-5
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- MEK inhibitor
Catalog No.:BCC1738
CAS No.:334951-92-7
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 873798-09-5 | SDF | Download SDF |
| PubChem ID | 11099301 | Appearance | Powder |
| Formula | C10H15BF4N6O | M.Wt | 322.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
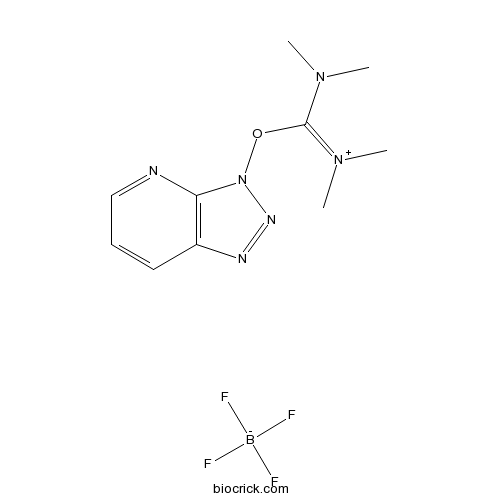
| Chemical Name | [dimethylamino(triazolo[4,5-b]pyridin-3-yloxy)methylidene]-dimethylazanium;tetrafluoroborate | ||
| SMILES | [B-](F)(F)(F)F.CN(C)C(=[N+](C)C)ON1C2=C(C=CC=N2)N=N1 | ||
| Standard InChIKey | AUPDFAPCZZXFMX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H15N6O.BF4/c1-14(2)10(15(3)4)17-16-9-8(12-13-16)6-5-7-11-9;2-1(3,4)5/h5-7H,1-4H3;/q+1;-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

TATU Dilution Calculator

TATU Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1046 mL | 15.5231 mL | 31.0463 mL | 62.0925 mL | 77.6156 mL |
| 5 mM | 0.6209 mL | 3.1046 mL | 6.2093 mL | 12.4185 mL | 15.5231 mL |
| 10 mM | 0.3105 mL | 1.5523 mL | 3.1046 mL | 6.2093 mL | 7.7616 mL |
| 50 mM | 0.0621 mL | 0.3105 mL | 0.6209 mL | 1.2419 mL | 1.5523 mL |
| 100 mM | 0.031 mL | 0.1552 mL | 0.3105 mL | 0.6209 mL | 0.7762 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
TATU
- PLX647
Catalog No.:BCC6370
CAS No.:873786-09-5
- Omecamtiv mecarbil
Catalog No.:BCC3710
CAS No.:873697-71-3
- Demethylcarolignan E
Catalog No.:BCN7018
CAS No.:873694-46-3
- GDC-0152
Catalog No.:BCC2252
CAS No.:873652-48-3
- Lupeolic acid
Catalog No.:BCN6520
CAS No.:87355-32-6
- PU-H71
Catalog No.:BCC1872
CAS No.:873436-91-0
- Triangularine
Catalog No.:BCN2051
CAS No.:87340-27-0
- Fortuneine
Catalog No.:BCN6401
CAS No.:87340-25-8
- TC-P 262
Catalog No.:BCC6155
CAS No.:873398-67-5
- 1,7-Dihydroxy-4-methoxyxanthone
Catalog No.:BCN7602
CAS No.:87339-76-2
- Ramipril
Catalog No.:BCC5012
CAS No.:87333-19-5
- AIM-100
Catalog No.:BCC1333
CAS No.:873305-35-2
- BMS-599626 Hydrochloride
Catalog No.:BCC1426
CAS No.:873837-23-1
- Fidaxomicin
Catalog No.:BCC4660
CAS No.:873857-62-6
- 3,4,5'-Trimethoxy-3',4'-methylenedioxy-7,9':7',9-diepoxylignan
Catalog No.:BCN7238
CAS No.:873867-94-8
- Neotriangularine
Catalog No.:BCN2052
CAS No.:87392-67-4
- 2,3-Dihydroamentoflavone 7,4'-dimethyl ether
Catalog No.:BCN1320
CAS No.:873999-88-3
- Denudatin B
Catalog No.:BCN4558
CAS No.:87402-88-8
- Euchrestaflavanone B
Catalog No.:BCN6838
CAS No.:87402-91-3
- RO4987655
Catalog No.:BCC5135
CAS No.:874101-00-5
- Daphnilongeranin A
Catalog No.:BCN4418
CAS No.:874201-05-5
- Liriopesides B
Catalog No.:BCN2811
CAS No.:87425-34-1
- Millewanin G
Catalog No.:BCN6461
CAS No.:874303-33-0
- Millewanin H
Catalog No.:BCN6465
CAS No.:874303-34-1
Crystal structure of the 1,3,6,8-tetra-aza-tri-cyclo[4.3.1.1(3,8)]undecane (TATU)-4-nitro-phenol (1/2) adduct: the role of anomeric effect in the formation of a second hydrogen-bond inter-action.[Pubmed:26594510]
Acta Crystallogr E Crystallogr Commun. 2015 Oct 24;71(Pt 11):1356-60.
In the title ternary co-crystalline adduct, C7H14N4.2C6H5NO3, mol-ecules are linked by two inter-molecular O-Hcdots, three dots, centeredN hydrogen bonds, forming a tricomponent aggregates in the asymmetric unit. The hydrogen-bond formation to one of the N atoms is enough to induce structural stereoelectronic effects in the normal donor-->acceptor direction. In the title adduct, the two independent nitro-phenol mol-ecules are essentially planar, with maximum deviations of 0.0157 (13) and 0.0039 (13) A. The dihedral angles between the planes of the nitro group and the attached benzene rings are 4.04 (17) and 5.79 (17) degrees . In the crystal, aggregates are connected by C-Hcdots, three dots, centeredO hydrogen bonds, forming a supra-molecular dimer enclosing an R 6 (6)(32) ring motif. Additional C-Hcdots, three dots, centeredO inter-molecular hydrogen-bonding inter-actions form a second supra-molecular inversion dimer with an R 2 (2)(10) motif. These units are linked via C-Hcdots, three dots, centeredO and C-Hcdots, three dots, centeredN hydrogen bonds, forming a three-dimensional network.
Mechanochemical synthesis and crystal structure of a 1:2 co-crystal of 1,3,6,8-tetra-aza-tri-cyclo[4.3.1.1(3,8)]undecane (TATU) and 4-chloro-3,5-dimethyl-phenol.[Pubmed:27840729]
Acta Crystallogr E Crystallogr Commun. 2016 Oct 25;72(Pt 11):1651-1653.
Solvent-free treatment of 1,3,6,8-tetra-aza-tri-cyclo-[4.3.1.1(3,8)]undecano (TATU) with 4-chloro-3,5-di-methyl-phenol led to the formation of the title co-crystal, C7H14N4.2C8H9ClO. The asymmetric unit contains one aminal cage mol-ecule and two phenol mol-ecules linked via two O-Hcdots, three dots, centeredN hydrogen bonds. In the aminal cage, the N-CH2-CH2-N unit is slightly distorted from a syn periplanar geometry. Aromatic pi-pi stacking between the benzene rings from two different neighbouring phenol mol-ecules [centroid-centroid distance = 4.0570 (11) A] consolidates the crystal packing.
Crystal structure of the 1:2 co-crystal of 1,3,6,8-tetra-aza-tri-cyclo-[4.3.1.1(3,8)]undecane (TATU) and 4-chloro-phenol (1/2).[Pubmed:27840728]
Acta Crystallogr E Crystallogr Commun. 2016 Oct 25;72(Pt 11):1648-1650.
In the title compound, C7H14N4.2C6H5ClO, which crystallized with two crystallographically independent 4-chloro-phenol mol-ecules and one 1,3,6,8-tetra-aza-tri-cyclo-[4.3.1.1(3,8)]undecane (TATU) mol-ecule in the asymmetric unit, the independent components are linked by two O-Hcdots, three dots, centeredN hydrogen bonds. The hydrogen-bond acceptor sites are two non-equivalent N atoms from the aminal cage structure, and the tricyclic system distorts by changing the C-N bond lengths. In the crystal, these hydrogen-bonded aggregates are linked into chains along the c axis by C-Hcdots, three dots, centeredN hydrogen bonds. The crystal structure also features C-Hcdots, three dots, centeredpi contacts.


