Liriopesides BCAS# 87425-34-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 87425-34-1 | SDF | Download SDF |
| PubChem ID | 21630160 | Appearance | Powder |
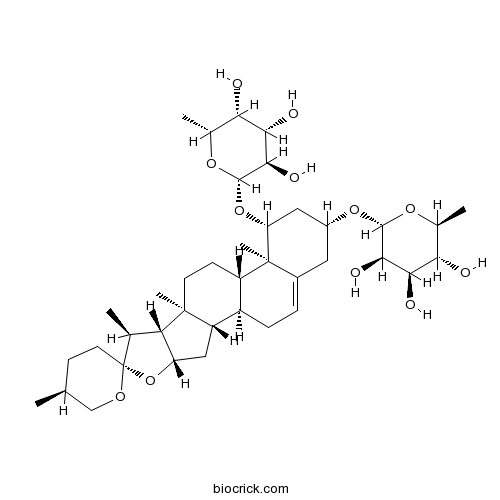
| Formula | C39H62O12 | M.Wt | 722.9 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Synonyms | Nolinospiroside F;Spicatoside B | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3R,4R,5R,6R)-2-methyl-6-[(1S,2S,4S,5'S,6R,7S,8R,9S,12S,13R,14R,16R)-5',7,9,13-tetramethyl-14-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyspiro[5-oxapentacyclo[10.8.0.02,9.04,8.013,18]icos-18-ene-6,2'-oxane]-16-yl]oxyoxane-3,4,5-triol | ||
| SMILES | CC1CCC2(C(C3C(O2)CC4C3(CCC5C4CC=C6C5(C(CC(C6)OC7C(C(C(C(O7)C)O)O)O)OC8C(C(C(C(O8)C)O)O)O)C)C)C)OC1 | ||
| Standard InChIKey | RMIQIULKBBCLIL-KJXSMTROSA-N | ||
| Standard InChI | InChI=1S/C39H62O12/c1-17-9-12-39(46-16-17)18(2)28-26(51-39)15-25-23-8-7-21-13-22(49-35-33(44)31(42)29(40)19(3)47-35)14-27(38(21,6)24(23)10-11-37(25,28)5)50-36-34(45)32(43)30(41)20(4)48-36/h7,17-20,22-36,40-45H,8-16H2,1-6H3/t17-,18-,19-,20+,22+,23+,24-,25-,26-,27+,28-,29-,30-,31+,32-,33+,34+,35-,36-,37-,38-,39+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Liriopesides B shows obvious antioxidant and bacteriostatic activities, it also exhibits potential anticancer activity against human ovarian cancer A2780 cells. |
| Targets | AKP | Antifection |
| In vitro | Liriopesides B inhibited cell growth and decreased CA125 level in human ovarian cancer A2780 cells.[Reference: WebLink]Natural Product Research, 2017:1-5.To study the effect of Liriopesides B on cell growth curve, cell doubling time, the activity of tumour marker CA125 and alkaline phosphatase (AKP) in human ovarian cancer A2780 cells. Study on Activities of Polysaccharide and Liriopesides B from Liriope Spicata Lour. var. Prolifera Y. T. Ma in Vitro.[Reference: WebLink]Food Research and Development, 2018.Polysaccharide fromLiriope Spicata Lour. var. Prolifera Y. T. Ma was extracted via hot water extraction and alcohol precipitation method,and its antioxidant activities in vitro were studied.
|

Liriopesides B Dilution Calculator

Liriopesides B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3833 mL | 6.9166 mL | 13.8332 mL | 27.6663 mL | 34.5829 mL |
| 5 mM | 0.2767 mL | 1.3833 mL | 2.7666 mL | 5.5333 mL | 6.9166 mL |
| 10 mM | 0.1383 mL | 0.6917 mL | 1.3833 mL | 2.7666 mL | 3.4583 mL |
| 50 mM | 0.0277 mL | 0.1383 mL | 0.2767 mL | 0.5533 mL | 0.6917 mL |
| 100 mM | 0.0138 mL | 0.0692 mL | 0.1383 mL | 0.2767 mL | 0.3458 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Daphnilongeranin A
Catalog No.:BCN4418
CAS No.:874201-05-5
- RO4987655
Catalog No.:BCC5135
CAS No.:874101-00-5
- Euchrestaflavanone B
Catalog No.:BCN6838
CAS No.:87402-91-3
- Denudatin B
Catalog No.:BCN4558
CAS No.:87402-88-8
- 2,3-Dihydroamentoflavone 7,4'-dimethyl ether
Catalog No.:BCN1320
CAS No.:873999-88-3
- Neotriangularine
Catalog No.:BCN2052
CAS No.:87392-67-4
- 3,4,5'-Trimethoxy-3',4'-methylenedioxy-7,9':7',9-diepoxylignan
Catalog No.:BCN7238
CAS No.:873867-94-8
- Fidaxomicin
Catalog No.:BCC4660
CAS No.:873857-62-6
- BMS-599626 Hydrochloride
Catalog No.:BCC1426
CAS No.:873837-23-1
- TATU
Catalog No.:BCC2822
CAS No.:873798-09-5
- PLX647
Catalog No.:BCC6370
CAS No.:873786-09-5
- Omecamtiv mecarbil
Catalog No.:BCC3710
CAS No.:873697-71-3
- Millewanin G
Catalog No.:BCN6461
CAS No.:874303-33-0
- Millewanin H
Catalog No.:BCN6465
CAS No.:874303-34-1
- Glepidotin B
Catalog No.:BCN4844
CAS No.:87440-56-0
- 6-Dehydroxy-8-hydroxygaleopsinolone
Catalog No.:BCN7402
CAS No.:87440-66-2
- Viteralone
Catalog No.:BCN4419
CAS No.:87440-75-3
- 11(13)-Dehydroivaxillin
Catalog No.:BCN4420
CAS No.:87441-73-4
- SEN 12333
Catalog No.:BCC6182
CAS No.:874450-44-9
- Schizanrin L
Catalog No.:BCN3620
CAS No.:874472-16-9
- (25RS)-Ruscogenin
Catalog No.:BCN7805
CAS No.:874485-32-2
- H-Arg-OtBu.2HCl
Catalog No.:BCC2862
CAS No.:87459-72-1
- Liriope muscari baily saponins C
Catalog No.:BCN2340
CAS No.:87480-46-4
- Dihydroajugapitin
Catalog No.:BCN4421
CAS No.:87480-84-0
A comparison of pectoralis versus lumbar skeletal muscle indices for defining sarcopenia in diffuse large B-cell lymphoma - two are better than one.[Pubmed:28388585]
Oncotarget. 2017 Jul 18;8(29):47007-47019.
BACKGROUNDS: Sarcopenia is known to be associated with poor clinical outcome in patients with diffuse large B-cell lymphoma (DLBCL). There is no consensus concerning the optimal method to define sarcopenia in DLBCL. METHODS: We retrospectively reviewed 193 DLBCL patients treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) therapy. Sarcopenia was classified by the region where the pretreatment skeletal muscle index (SMI) was measured. RESULTS: Both the sarcopenia-L3 and sarcopenia-pectoralis muscle (PM) groups had increased incidences of severe treatment-related toxicities and treatment discontinuation compared with the non-sarcopenia-L3 and non-sarcopenia-PM groups, respectively. The sarcopenia-L3 and non-sarcopenia-L3 groups had 5-year overall survival (OS) rates of 40.5% and 67.8% (p < 0.001), respectively. The sarcopenia-PM and non-sarcopenia-PM groups had 5-year OS rates of 35.9% and 69.0% (p < 0.001), respectively. When the sarcopenia-L3 alone and sarcopenia-PM alone groups were compared, there were no differences in baseline characteristics, treatment toxicity, or survival. In multivariate analysis, when compared with the non-sarcopenia-both group, OS was significantly worse in the sarcopenia-both group (HR, 2.480; 95% CI, 1.284 - 4.792; p = 0.007), but not in patients with either sarcopenia-L3 alone or sarcopenia-PM alone (p = 0.151). CONCLUSIONS: L3- and PM-SMIs are equally useful to define sarcopenia, which is related to intolerance to R-CHOP therapy and to worse survival in patients with DLBCL. More prognostic information can be obtained when these two SMIs are combined to define sarcopenia.
Publisher's Note: Measurement of the CP Asymmetry in B_{s}^{0}-B[over ]_{s}^{0} Mixing [Phys. Rev. Lett. 117, 061803 (2016)].[Pubmed:28388175]
Phys Rev Lett. 2017 Mar 24;118(12):129903.
This corrects the article DOI: 10.1103/PhysRevLett.117.061803.
Prognostic significance of the red blood cell distribution width in diffuse large B-cell lymphoma patients.[Pubmed:28388534]
Oncotarget. 2017 Jun 20;8(25):40724-40731.
This study examined the prognostic value of the baseline red blood cell distribution width (RDW) in diffuse large B cell lymphoma (DLBCL) patients. The associations between RDW and clinical characteristics were assessed in 161 DLBCL patients from 2005 to 2016. The log-rank test, univariate analysis, and Cox regression analysis were used to evaluate the relationship between RDW and survival. A RDW of 14.1% was considered to be the optimal cut-off value for predicting prognosis. A high RDW was associated with more frequent B symptoms (P=0.001), a higher International Prognostic Index score (P=0.032), more extranodal sites of disease (P=0.035), and significantly lower Eastern Cooperative Oncology Group performance status (P=0.031). The log-rank test demonstrated that patients with a high RDW had a shorter overall survival (OS) (2-year OS rate, 53.6% vs. 83.6%, P<0.001) and progression-free survival (PFS) (2-year PFS rate, 44.7% vs. 81.8%, P<0.001). The multivariate analysis demonstrated that RDW >/=14.1% was an independent predictor of OS (odds ratio [OR] = 0.345, P<0.001) and PFS (OR = 0.393, P=0.001). We demonstrated that a high RDW predicted an unfavorable prognosis in patients with DLBCL.
Immune balance in Hepatitis B Infection: Present and Future Therapies.[Pubmed:28387980]
Scand J Immunol. 2017 Jul;86(1):4-14.
Chronic hepatitis B virus (HBV) infection affects millions of people worldwide and about half a million people die every year. India represents the second largest pool of chronic HBV infections with an estimated 40 million chronically infected patients. Persistence or clearance of HBV infection mainly depends upon host immune responses. Chronically infected individuals remain in immune tolerant phase unless HBV flares and leads to the development of chronic active hepatitis or acute-on-chronic liver failure. Strategies based on inhibition of viral replication (nucleoside analogues) or immune modulation (interferons) as monotherapy, or in combination in sequential therapies, are currently being used globally for reducing HBV viral load and mediating HBsAg clearance. However, the immune status and current therapies for promoting sustained virological responses in HBV-infected patients remain suboptimal. Elimination of cccDNA is major challenge for future therapies, and new molecules such as NTCP, Toll-like receptor (TLR)7 agonist (GS9620) and cyclophilin have emerged as potential targets for preventing HBV entry and replication. Other than these, HBV cccDNA elimination is the major target for future therapies.


