AZD4547FGFR inhibitor CAS# 1035270-39-3 |
- LY2874455
Catalog No.:BCC1723
CAS No.:1254473-64-7
- PD 173074
Catalog No.:BCC3662
CAS No.:219580-11-7
- Nintedanib (BIBF 1120)
Catalog No.:BCC3661
CAS No.:656247-17-5
- SSR128129E
Catalog No.:BCC4498
CAS No.:848318-25-2
- BGJ398
Catalog No.:BCC1278
CAS No.:872511-34-7
- XL228
Catalog No.:BCC2058
CAS No.:898280-07-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1035270-39-3 | SDF | Download SDF |
| PubChem ID | 51039095 | Appearance | Powder |
| Formula | C26H33N5O3 | M.Wt | 463.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (269.65 mM; Need ultrasonic) | ||
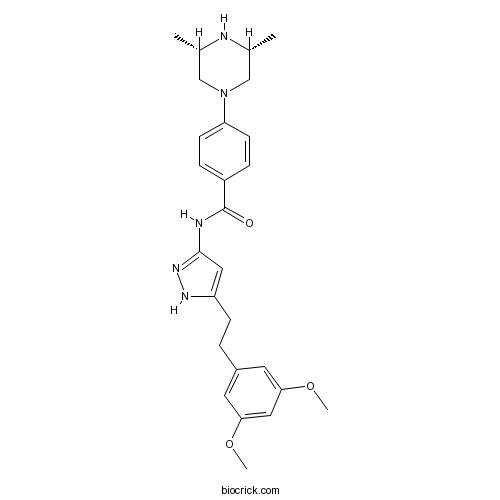
| Chemical Name | N-[5-[2-(3,5-dimethoxyphenyl)ethyl]-1H-pyrazol-3-yl]-4-[(3R,5S)-3,5-dimethylpiperazin-1-yl]benzamide | ||
| SMILES | CC1CN(CC(N1)C)C2=CC=C(C=C2)C(=O)NC3=NNC(=C3)CCC4=CC(=CC(=C4)OC)OC | ||
| Standard InChIKey | VRQMAABPASPXMW-HDICACEKSA-N | ||
| Standard InChI | InChI=1S/C26H33N5O3/c1-17-15-31(16-18(2)27-17)22-9-6-20(7-10-22)26(32)28-25-13-21(29-30-25)8-5-19-11-23(33-3)14-24(12-19)34-4/h6-7,9-14,17-18,27H,5,8,15-16H2,1-4H3,(H2,28,29,30,32)/t17-,18+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AZD4547 is a novel selective inhibitor of FGFR for FGFR1/2/3 with IC50 of 0.2 nM/2.5 nM/1.8 nM, weaker activity against FGFR4, VEGFR2(KDR), and little activity observed against IGFR, CDK2, and p38. | |||||
| Targets | FGFR1 | FGFR2 | FGFR3 | |||
| IC50 | 0.2 nM | 2.5 nM | 1.8 nM | |||

AZD4547 Dilution Calculator

AZD4547 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1572 mL | 10.7859 mL | 21.5717 mL | 43.1434 mL | 53.9293 mL |
| 5 mM | 0.4314 mL | 2.1572 mL | 4.3143 mL | 8.6287 mL | 10.7859 mL |
| 10 mM | 0.2157 mL | 1.0786 mL | 2.1572 mL | 4.3143 mL | 5.3929 mL |
| 50 mM | 0.0431 mL | 0.2157 mL | 0.4314 mL | 0.8629 mL | 1.0786 mL |
| 100 mM | 0.0216 mL | 0.1079 mL | 0.2157 mL | 0.4314 mL | 0.5393 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AZD4547 is a potent, specific, orally bioavailable fibroblast growth factors receptor (FGFR) tyrosine kinase inhibitor. It inhibits FGFR1, FGFR2, and FGFR3 with IC50 values of 12, 2 and 40 nM, respectively. AZD4547 has also been reported to block FGFR1, FGFR2, and FGFR3 autophosphorylation with IC50 values of 0.2, 2.5, and 1.8 nM, respectively [1].
AZD4547 has been demonstrated to inhibit cell proliferation and inhibit FGFRs and their downstream markers including PLCg and FRS2 phosphorylation in breast cell line Sum52-PE (expressing wild-type FGFR2), multiple myeloma line KMS11 (expressing Y373C mutated FGFR3 protein) and acute myeloid leukemia cell line KG1a (expressing wild-type FGFR1) [1].
Anti-carcinoma effect of AZD4547 has been observed in lung cancer xeonograft mice orally treated with AZD4547. This effect is believed to be via the specifically inhibition of the activity of FGFR but not kinase insert domain receptor (KDR)[1].
References:
[1] Gavine PR1,?Mooney L,?Kilgour E,?Thomas AP,?Al-Kadhimi K,?Beck S,?Rooney C,?Coleman T,?Baker D,?Mellor MJ,?Brooks AN,?Klinowska T. AZD4547: an orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res.?2012 Apr 15;72(8):2045-56.
- AZ505 ditrifluoroacetate
Catalog No.:BCC4265
CAS No.:1035227-44-1
- AZ505
Catalog No.:BCC4264
CAS No.:1035227-43-0
- Phyllanthin
Catalog No.:BCN5848
CAS No.:10351-88-9
- 9-Dehydroandrostenedione
Catalog No.:BCC8801
CAS No.:1035-69-4
- Fmoc-D-N-Me-Leu-OH
Catalog No.:BCC3346
CAS No.:103478-63-3
- Fmoc-N-Me-Leu-OH
Catalog No.:BCC3345
CAS No.:103478-62-2
- Fmoc-D-N- Me-Val-OH
Catalog No.:BCC3359
CAS No.:103478-58-6
- Diacetylpiptocarphol
Catalog No.:BCN4737
CAS No.:103476-99-9
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- Maprotiline HCl
Catalog No.:BCC4329
CAS No.:10347-81-6
- NMS-1286937
Catalog No.:BCC5358
CAS No.:1034616-18-6
- Xanthone V1a
Catalog No.:BCN7922
CAS No.:103450-96-0
- Apiosylskimmin
Catalog No.:BCN2455
CAS No.:103529-94-8
- Huperzine B
Catalog No.:BCN1059
CAS No.:103548-82-9
- 3,7-O-Diacetylpinobanksin
Catalog No.:BCN5849
CAS No.:103553-98-6
- TAK-733
Catalog No.:BCC4587
CAS No.:1035555-63-5
- Lansoprazole
Catalog No.:BCC1058
CAS No.:103577-45-3
- P005672 hydrochloride
Catalog No.:BCC6406
CAS No.:1035979-44-2
- Isookanin
Catalog No.:BCN6476
CAS No.:1036-49-3
- 5,7-Dimethoxyflavanone
Catalog No.:BCN3569
CAS No.:1036-72-2
- RETRA hydrochloride
Catalog No.:BCC2415
CAS No.:1036069-26-7
- Janolusimide
Catalog No.:BCN1840
CAS No.:103612-45-9
- H-Phe(2-Cl)-OH
Catalog No.:BCC3165
CAS No.:103616-89-3
- (±)-5'-Chloro-5'-deoxy-ENBA
Catalog No.:BCC7716
CAS No.:103626-26-2
Safety, tolerability and pharmacokinetics of the fibroblast growth factor receptor inhibitor AZD4547 in Japanese patients with advanced solid tumours: a Phase I study.[Pubmed:28070720]
Invest New Drugs. 2017 Aug;35(4):451-462.
Background AZD4547 is a potent, oral, highly selective fibroblast growth factor receptor (FGFR) inhibitor in clinical development for treating tumours with a range of FGFR aberrations, including FGFR mutations, amplifications and fusions. Methods This open-label, Phase I, multicentre study (NCT01213160) evaluated the safety, pharmacokinetics, and preliminary antitumour efficacy (RECIST v1.1) of AZD4547 monotherapy in Japanese patients with advanced solid tumours. Part A was a dose-escalation part; Part B was a dose-expansion part in patients with FGFR-amplified tumours, confirmed by fluorescence in situ hybridization. Results Thirty patients enrolled in Part A (dose range: 40 mg twice daily [bid] to 120 mg bid; 160 mg once daily [qd]), four in Part B (80 mg bid). No dose-limiting toxicities were observed and maximum tolerated dose was not determined. Most common adverse events (AEs; any grade) were: dysgeusia (50% of patients); stomatitis (41%); diarrhoea (38%); hyperphosphataemia (38%); dry mouth (35%). Common grade >/=3 AEs were nausea (12% of patients) and neutropenia (9%). No complete or partial responses were observed: 21/30 patients had stable disease >/=4 weeks in Part A, and 1/4 patients had stable disease >/=10 weeks in Part B. Following single and multiple dosing, absorption rate appeared moderate; peak plasma concentrations generally occurred 3-4 h post-dose, then declined biphasically with terminal half-life ~30 h. Steady state was reached by day 8. Compared with single dosing, plasma concentrations were, on average, 2.4- and 3.3- to 5.4-fold higher after qd and bid dosing, respectively. Conclusions AZD4547 was well tolerated in Japanese patients, with best response of stable disease >/=4 weeks.
Activity of fibroblast growth factor receptor inhibitors TKI258, ponatinib and AZD4547 against TPRFGFR1 fusion.[Pubmed:28138694]
Mol Med Rep. 2017 Mar;15(3):1024-1030.
8p11 myeloproliferative syndrome (EMS) is a rare disease characterized by the constitutive activation of fibroblast growth factor receptor 1 (FGFR1). To date, four cases of EMS with the chromosomal translocation, t(1;8)(q25;p11.2), have been reported. In the present study, TPRFGFR1expressing Baf3 cells were established and confirmed by polymerase chain reaction. To identify the most promising drug for EMS, the activities and associated mechanism of three tyrosine kinase inhibitors (TKIs), TKI258, ponatinib and AZD4547, against TPRFGFR1 were tested by MTT assay, flow cytometry and western blot. The data demonstrated that TPRFGFR1 was localized in the cytoplasm, and was able to transform interleukin-3-dependent hematopoietic Baf3 cells into growth factorindependent cells. All of the three TKIs markedly inhibited the proliferation of TPRFGFR1expressing Baf3 cells, and the activation of FGFR1 and the downstream signaling molecules, extracellular signalregulated kinase 1/2, phospholipiase Cgamma and signal transducer and activator of transcription 5. AZD4547 was the most efficient drug, and TKI258 was the least. By contrast, no significant difference was found among the three drugs on their effect on cell apoptosis. Taken together, the data obtained in the present study suggested that AZD4547 had increased potency, compared with TKI258 and ponatinib, for the treatment of EMS.
Insight into resistance mechanisms of AZD4547 and E3810 to FGFR1 gatekeeper mutation via theoretical study.[Pubmed:28255231]
Drug Des Devel Ther. 2017 Feb 17;11:451-461.
Inhibitors targeting the amplification of the fibroblast growth factor receptor 1 (FGFR1) have found success in the treatment of FGFR1-positive squamous cell lung and breast cancers. A secondary mutation of gatekeeper residue (V561M) in the binding site has been linked to the acquired resistance. Recently, two well-known small molecule inhibitors of FGFR1, AZD4547 and E3810, reported that the V561M mutation confers significant resistance to E3810, while retaining affinity for AZD4547. FGFR1 is widely investigated as potential therapeutic target, while there are few computational studies made to understand the resistance mechanisms about FGFR1 V561M gatekeeper mutation. In this study, molecular docking, classical molecular dynamics simulations, molecular mechanics/generalized born surface area (MM/GBSA) free energy calculations, and umbrella sampling (US) simulations were carried out to make clear the principle of the binding preference of AZD4547 and E3810 toward FGFR1 V561M gatekeeper mutation. The results provided by MM/GBSA reveal that AZD4547 has similar binding affinity to both FGFR1(WT) and FGFR1(V561M), whereas E3810 has much higher binding affinity to FGFR1(WT) than to FGFR1(V561M). Comparison of individual energy terms indicates that the major variation of E3810 between FGFR1(WT) and FGFR1(V561M) are van der Waals interactions. In addition, US simulations prove that the potential of mean force (PMF) profile of AZD4547 toward FGFR1(WT) and FGFR1(V561M) has similar PMF depth. However, the PMF profile of E3810 toward FGFR1(WT) and FGFR1(V561M) has much higher PMF depth, suggesting that E3810 is more easily dissociated from FGFR1(V561M) than from FGFR1(WT). The results not only show the drug-resistance determinants of FGFR1 gatekeeper mutation but also provide valuable implications and provide vital clues for the development of new inhibitors to combat drug resistance.
Identification of Pharmacodynamic Transcript Biomarkers in Response to FGFR Inhibition by AZD4547.[Pubmed:27550940]
Mol Cancer Ther. 2016 Nov;15(11):2802-2813.
The challenge of developing effective pharmacodynamic biomarkers for preclinical and clinical testing of FGFR signaling inhibition is significant. Assays that rely on the measurement of phospho-protein epitopes can be limited by the availability of effective antibody detection reagents. Transcript profiling enables accurate quantification of many biomarkers and provides a broader representation of pathway modulation. To identify dynamic transcript biomarkers of FGFR signaling inhibition by AZD4547, a potent inhibitor of FGF receptors 1, 2, and 3, a gene expression profiling study was performed in FGFR2-amplified, drug-sensitive tumor cell lines. Consistent with known signaling pathways activated by FGFR, we identified transcript biomarkers downstream of the RAS-MAPK and PI3K/AKT pathways. Using different tumor cell lines in vitro and xenografts in vivo, we confirmed that some of these transcript biomarkers (DUSP6, ETV5, YPEL2) were modulated downstream of oncogenic FGFR1, 2, 3, whereas others showed selective modulation only by FGFR2 signaling (EGR1). These transcripts showed consistent time-dependent modulation, corresponding to the plasma exposure of AZD4547 and inhibition of phosphorylation of the downstream signaling molecules FRS2 or ERK. Combination of FGFR and AKT inhibition in an FGFR2-mutated endometrial cancer xenograft model enhanced modulation of transcript biomarkers from the PI3K/AKT pathway and tumor growth inhibition. These biomarkers were detected on the clinically validated nanoString platform. Taken together, these data identified novel dynamic transcript biomarkers of FGFR inhibition that were validated in a number of in vivo models, and which are more robustly modulated by FGFR inhibition than some conventional downstream signaling protein biomarkers. Mol Cancer Ther; 15(11); 2802-13. (c)2016 AACR.


