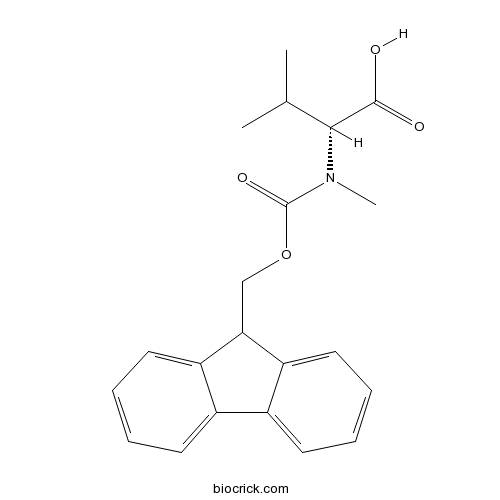
Fmoc-D-N- Me-Val-OHCAS# 103478-58-6 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103478-58-6 | SDF | Download SDF |
| PubChem ID | 16213159 | Appearance | Powder |
| Formula | C21H23NO4 | M.Wt | 353.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R)-2-[9H-fluoren-9-ylmethoxycarbonyl(methyl)amino]-3-methylbutanoic acid | ||
| SMILES | CC(C)C(C(=O)O)N(C)C(=O)OCC1C2=CC=CC=C2C3=CC=CC=C13 | ||
| Standard InChIKey | YCXXXPZNQXXRIG-LJQANCHMSA-N | ||
| Standard InChI | InChI=1S/C21H23NO4/c1-13(2)19(20(23)24)22(3)21(25)26-12-18-16-10-6-4-8-14(16)15-9-5-7-11-17(15)18/h4-11,13,18-19H,12H2,1-3H3,(H,23,24)/t19-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-D-N- Me-Val-OH Dilution Calculator

Fmoc-D-N- Me-Val-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8297 mL | 14.1483 mL | 28.2965 mL | 56.5931 mL | 70.7414 mL |
| 5 mM | 0.5659 mL | 2.8297 mL | 5.6593 mL | 11.3186 mL | 14.1483 mL |
| 10 mM | 0.283 mL | 1.4148 mL | 2.8297 mL | 5.6593 mL | 7.0741 mL |
| 50 mM | 0.0566 mL | 0.283 mL | 0.5659 mL | 1.1319 mL | 1.4148 mL |
| 100 mM | 0.0283 mL | 0.1415 mL | 0.283 mL | 0.5659 mL | 0.7074 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-D-N- Me-Val-OH
- Diacetylpiptocarphol
Catalog No.:BCN4737
CAS No.:103476-99-9
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- Maprotiline HCl
Catalog No.:BCC4329
CAS No.:10347-81-6
- NMS-1286937
Catalog No.:BCC5358
CAS No.:1034616-18-6
- Xanthone V1a
Catalog No.:BCN7922
CAS No.:103450-96-0
- Z-Cyclopentyl-AP4
Catalog No.:BCC7616
CAS No.:103439-17-4
- CTAP
Catalog No.:BCC5776
CAS No.:103429-32-9
- CTOP
Catalog No.:BCC5780
CAS No.:103429-31-8
- Devazepide
Catalog No.:BCC7319
CAS No.:103420-77-5
- Disodium (R)-2-Hydroxyglutarate
Catalog No.:BCC6515
CAS No.:103404-90-6
- Octyl gallate
Catalog No.:BCN8432
CAS No.:1034-01-1
- 1-O-Methylnataloe-emodin
Catalog No.:BCN7036
CAS No.:103392-51-4
- Fmoc-N-Me-Leu-OH
Catalog No.:BCC3345
CAS No.:103478-62-2
- Fmoc-D-N-Me-Leu-OH
Catalog No.:BCC3346
CAS No.:103478-63-3
- 9-Dehydroandrostenedione
Catalog No.:BCC8801
CAS No.:1035-69-4
- Phyllanthin
Catalog No.:BCN5848
CAS No.:10351-88-9
- AZ505
Catalog No.:BCC4264
CAS No.:1035227-43-0
- AZ505 ditrifluoroacetate
Catalog No.:BCC4265
CAS No.:1035227-44-1
- AZD4547
Catalog No.:BCC3711
CAS No.:1035270-39-3
- Apiosylskimmin
Catalog No.:BCN2455
CAS No.:103529-94-8
- Huperzine B
Catalog No.:BCN1059
CAS No.:103548-82-9
- 3,7-O-Diacetylpinobanksin
Catalog No.:BCN5849
CAS No.:103553-98-6
- TAK-733
Catalog No.:BCC4587
CAS No.:1035555-63-5
- Lansoprazole
Catalog No.:BCC1058
CAS No.:103577-45-3
Peptides from chiral C alpha,alpha-disubstituted glycines. Crystallographic characterization of conformation of C alpha-methyl, C alpha-isopropylglycine [(alpha Me)Val] in simple derivatives and model peptides.[Pubmed:1917310]
Int J Pept Protein Res. 1991 Jun;37(6):521-7.
The molecular and crystal structures of one derivative and three model peptides (to the pentapeptide level) of the chiral C alpha,alpha-disubstituted glycine C alpha-methyl, C alpha-isopropylglycine [(alpha Me)Val] have been determined by X-ray diffraction. The derivative is mClAc-L-(alpha Me)Val-OH, and the peptides are Z-L-(alpha Me)Val-(L-Ala)2-OMe monohydrate, Z-Aib-L-(alpha Me)Val-(Aib)2-OtBu, and Ac-(Aib)2-L-(alpha Me)Val-(Aib)2OtBu acetonitrile solvate. The tripeptide adopts a type-I beta-turn conformation stabilized by a 1----4N--H...O = C intramolecular H-bond. The tetra- and pentapeptides are folded in regular right-handed 3(10)-helices. All four L-(alpha Me)Val residues prefer phi, psi angles in the right-handed helical region of the conformational map. The results indicate that: (i) the (alpha Me)Val residue is a strong type-I/III beta-turn and helix former, and (ii) the relationship between (alpha Me)Val chirality and helix screw sense is the same as that of C alpha-monosubstituted protein amino-acids. The implications for the use of the (alpha Me)Val residue in designing conformationally constrained analogues of bioactive peptides are briefly discussed.
Formation of Fmoc-beta-alanine during Fmoc-protections with Fmoc-OSu.[Pubmed:18219706]
J Pept Sci. 2008 Jun;14(6):763-6.
During the Fmoc-protection of H-alpha-Me-Val-OH, an unknown side product was found and isolated. The characterization using various analytical methods led unambiguously to the result that Fmoc-beta-Ala-OH was formed during the reaction. The reagent Fmoc-OSu was proven to be the source of Fmoc-beta-Ala-OH, following a mechanism that involved many deprotonation and elimination steps and a Lossen-type rearrangement as key sequence. The impurity Fmoc-beta-Ala-OH was found in a variety of reactions in which Fmoc-OSu was applied, either in the reaction mixture or as a contamination of the crude product. Purification of the Fmoc-amino acid derivatives from this impurity incurred high costs and significant reductions in yield.


