CTAPSelective and potent μ antagonist CAS# 103429-32-9 |
- Stattic
Catalog No.:BCC1176
CAS No.:19983-44-9
- Fludarabine
Catalog No.:BCC2518
CAS No.:21679-14-1
- Cucurbitacin I
Catalog No.:BCC2439
CAS No.:2222-07-3
- WP1066
Catalog No.:BCC2194
CAS No.:857064-38-1
- SD 1008
Catalog No.:BCC2442
CAS No.:960201-81-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103429-32-9 | SDF | Download SDF |
| PubChem ID | 10418702 | Appearance | Powder |
| Formula | C51H69N13O11S2 | M.Wt | 1104.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
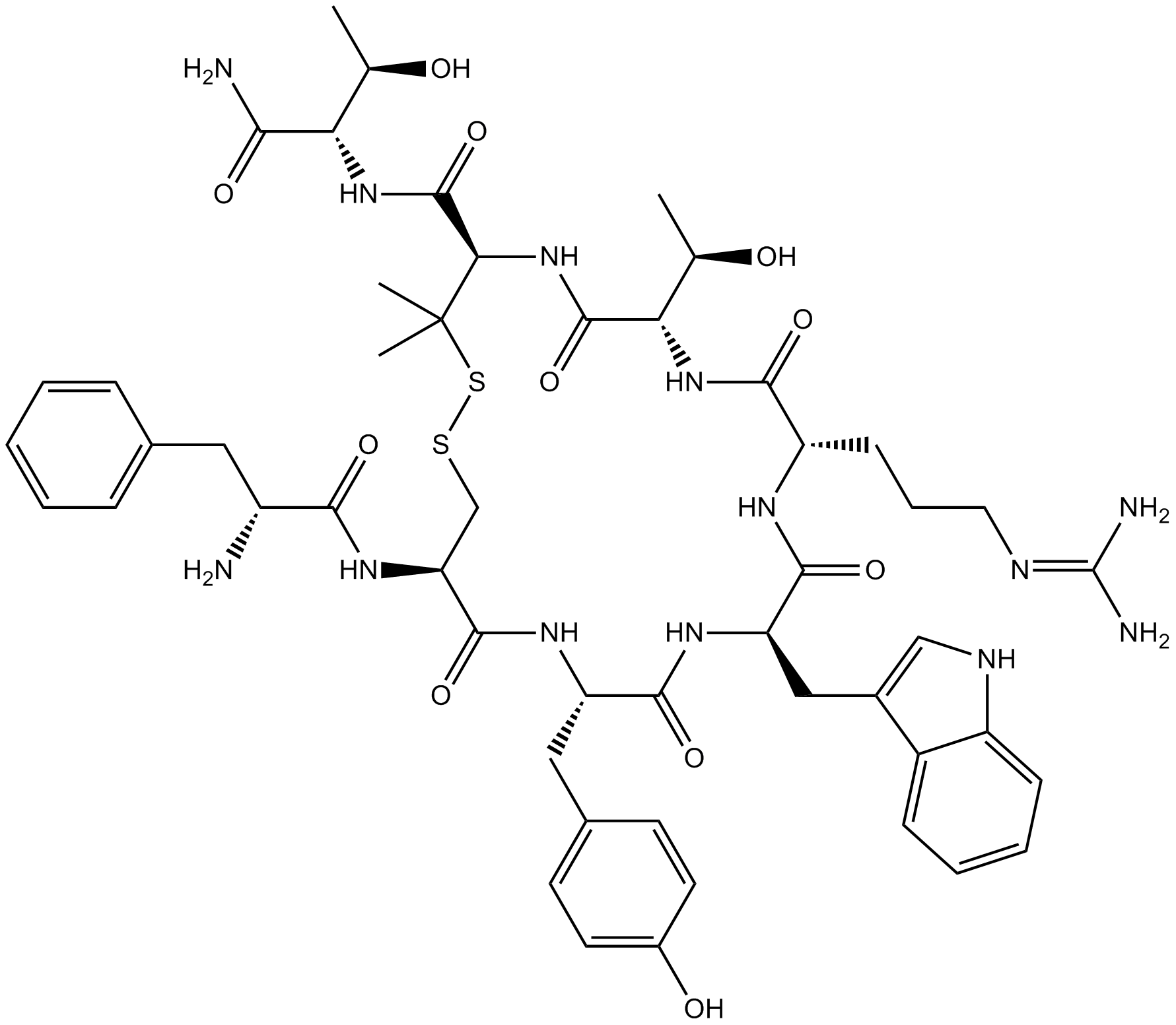
| Sequence | FCYWRTXT (Modifications: Phe-1 = D-Phe, Trp-4 = D-Trp, X = Pen, Disulfide bridge between 2 - 7, Thr-8 = C-terminal amide) | ||
| Chemical Name | (4R,7S,10S,13R,16S,19R)-N-[(2S,3R)-1-amino-3-hydroxy-1-oxobutan-2-yl]-19-[[(2R)-2-amino-3-phenylpropanoyl]amino]-10-[3-(diaminomethylideneamino)propyl]-7-[(1R)-1-hydroxyethyl]-16-[(4-hydroxyphenyl)methyl]-13-(1H-indol-3-ylmethyl)-3,3-dimethyl-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxamide | ||
| SMILES | CC(C1C(=O)NC(C(SSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCN=C(N)N)CC2=CNC3=CC=CC=C32)CC4=CC=C(C=C4)O)NC(=O)C(CC5=CC=CC=C5)N)(C)C)C(=O)NC(C(C)O)C(=O)N)O | ||
| Standard InChIKey | OFMQLVRLOGHAJI-FGHAYEPSSA-N | ||
| Standard InChI | InChI=1S/C51H69N13O11S2/c1-26(65)39(42(53)68)62-49(75)41-51(3,4)77-76-25-38(61-43(69)33(52)21-28-11-6-5-7-12-28)47(73)59-36(22-29-16-18-31(67)19-17-29)45(71)60-37(23-30-24-57-34-14-9-8-13-32(30)34)46(72)58-35(15-10-20-56-50(54)55)44(70)63-40(27(2)66)48(74)64-41/h5-9,11-14,16-19,24,26-27,33,35-41,57,65-67H,10,15,20-23,25,52H2,1-4H3,(H2,53,68)(H,58,72)(H,59,73)(H,60,71)(H,61,69)(H,62,75)(H,63,70)(H,64,74)(H4,54,55,56)/t26-,27-,33-,35+,36+,37-,38+,39+,40+,41-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective μ opioid receptor antagonist (IC50 = 3.5 nM). Displays > 1200-fold selectivity over δ opioid and somatostatin receptors. Brain penetrant and active in vivo. |

CTAP Dilution Calculator

CTAP Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CTOP
Catalog No.:BCC5780
CAS No.:103429-31-8
- Devazepide
Catalog No.:BCC7319
CAS No.:103420-77-5
- Disodium (R)-2-Hydroxyglutarate
Catalog No.:BCC6515
CAS No.:103404-90-6
- Octyl gallate
Catalog No.:BCN8432
CAS No.:1034-01-1
- 1-O-Methylnataloe-emodin
Catalog No.:BCN7036
CAS No.:103392-51-4
- HPGDS inhibitor 1
Catalog No.:BCC4065
CAS No.:1033836-12-2
- Telotristat
Catalog No.:BCC5128
CAS No.:1033805-28-5
- Salidroside
Catalog No.:BCN5966
CAS No.:10338-51-9
- GNF-5837
Catalog No.:BCC3668
CAS No.:1033769-28-6
- Itol A
Catalog No.:BCN5847
CAS No.:1033747-78-2
- GNE-493
Catalog No.:BCC8048
CAS No.:1033735-94-2
- 1-Methyl-L-4,5-dihydroorotic acid
Catalog No.:BCC8472
CAS No.:103365-69-1
- Z-Cyclopentyl-AP4
Catalog No.:BCC7616
CAS No.:103439-17-4
- Xanthone V1a
Catalog No.:BCN7922
CAS No.:103450-96-0
- NMS-1286937
Catalog No.:BCC5358
CAS No.:1034616-18-6
- Maprotiline HCl
Catalog No.:BCC4329
CAS No.:10347-81-6
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- Diacetylpiptocarphol
Catalog No.:BCN4737
CAS No.:103476-99-9
- Fmoc-D-N- Me-Val-OH
Catalog No.:BCC3359
CAS No.:103478-58-6
- Fmoc-N-Me-Leu-OH
Catalog No.:BCC3345
CAS No.:103478-62-2
- Fmoc-D-N-Me-Leu-OH
Catalog No.:BCC3346
CAS No.:103478-63-3
- 9-Dehydroandrostenedione
Catalog No.:BCC8801
CAS No.:1035-69-4
- Phyllanthin
Catalog No.:BCN5848
CAS No.:10351-88-9
- AZ505
Catalog No.:BCC4264
CAS No.:1035227-43-0
Role of platelet chemokines, PF-4 and CTAP-III, in cancer biology.[Pubmed:23800319]
J Hematol Oncol. 2013 Jun 24;6:42.
With the recent addition of anti-angiogenic agents to cancer treatment, the angiogenesis regulators in platelets are gaining importance. Platelet factor 4 (PF-4/CXCL4) and Connective tissue activating peptide III (CTAP-III) are two platelet-associated chemokines that modulate tumor angiogenesis, inflammation within the tumor microenvironment, and in turn tumor growth. Here, we review the role of PF-4 and CTAP-III in the regulation of tumor angiogenesis; the results of clinical trial using recombinant PF-4 (rPF-4); and the use of PF-4 and CTAP-III as cancer biomarkers.
Elevated levels of CXC chemokine connective tissue activating peptide (CTAP)-III in lung cancer patients.[Pubmed:21654877]
Am J Transl Res. 2011 May 15;3(3):226-33. Epub 2011 Apr 2.
Despite advances in treatments, lung cancer has been the leading cause of cancer-related deaths in the United States for the past several decades. Recent findings from the National Lung Screening Trial reveal that low-dose helical computed tomography (CT) scan screening of high-risk individuals reduces lung cancer mortality. This suggests that early detection is of key importance to improving patient outcome. However, of those screened with CT scans, 25% had positive scans that require further follow-up studies which often involve more radiation exposure and invasive tests to reduce false positive results. The purpose of this study was to identify candidate plasma biomarkers to aid in diagnosis of lung cancer in at-risk individuals. We found increased expression of the CXC chemokine connective tissue-activating peptide (CTAP)-III from plasma specimens of lung cancer patients compared to at-risk control subjects. Identification of the peptide was confirmed by the addition of an anti-NAP-2 antibody that recognizes CTAP-III and NAP-2. We also quantified and verified the increased levels of plasma CTAP-III with ELISA in patients with lung cancer (mean +/- SD, 1859 +/- 1219 ng/mL) compared to controls (698 +/- 434 ng/mL; P<0.001). Our findings demonstrate elevated plasma levels of CTAP-III occur in lung cancer patients. Further studies are required to determine if this chemokine could be utilized in a blood-based biomarker panel for the diagnosis of lung cancer.
The Contextualized Technology Adaptation Process (CTAP): Optimizing Health Information Technology to Improve Mental Health Systems.[Pubmed:25677251]
Adm Policy Ment Health. 2016 May;43(3):394-409.
Health information technologies have become a central fixture in the mental healthcare landscape, but few frameworks exist to guide their adaptation to novel settings. This paper introduces the contextualized technology adaptation process (CTAP) and presents data collected during Phase 1 of its application to measurement feedback system development in school mental health. The CTAP is built on models of human-centered design and implementation science and incorporates repeated mixed methods assessments to guide the design of technologies to ensure high compatibility with a destination setting. CTAP phases include: (1) Contextual evaluation, (2) Evaluation of the unadapted technology, (3) Trialing and evaluation of the adapted technology, (4) Refinement and larger-scale implementation, and (5) Sustainment through ongoing evaluation and system revision. Qualitative findings from school-based practitioner focus groups are presented, which provided information for CTAP Phase 1, contextual evaluation, surrounding education sector clinicians' workflows, types of technologies currently available, and influences on technology use. Discussion focuses on how findings will inform subsequent CTAP phases, as well as their implications for future technology adaptation across content domains and service sectors.
Blood-brain barrier permeability and bioavailability of a highly potent and mu-selective opioid receptor antagonist, CTAP: comparison with morphine.[Pubmed:8996221]
J Pharmacol Exp Ther. 1997 Jan;280(1):402-9.
D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) is a cyclic, penicillamine-containing oCTAPeptide that is structurally similar to somatostatin and displays greater antagonist potency and selectivity for mu-opioid receptors, compared with the classical mu-selective antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2. The aim of this study was to determine whether CTAP can enter the central nervous system (CNS) by crossing either the blood-brain barrier or the blood-cerebrospinal fluid barrier (CSF) and to characterize the mechanism of CNS entry. CNS entry of [3H]CTAP was compared with that of the vascular space marker [14C]inulin and the mu-agonist [3H]morphine. By using an in situ brain perfusion technique coupled to high-performance liquid chromatographic analysis, greater amounts of radioactivity were detected in the brain or CSF at most time points for [3H]CTAP, compared with [14C]inulin. [3H]CTAP was found to remain predominantly intact in the brain after a 20-min rat brain perfusion (62.8%). CTAP was also stable in the blood and serum of rats (T1/2 > 500 min), showing that the structure of this peptide offers enzymatic resistance. Additionally, [3H]CTAP was found to be extensively protein-bound to albumin in the perfusion medium (68.2%) and to proteins in rat serum (84.2%). Entry into the brain and CSF was not inhibited by the addition of unlabeled CTAP to the perfusion medium, suggesting that passage into the CNS is most likely through diffusion across the membranes that comprise the blood-brain barrier, rather than by saturable transport. Also, greater amounts of [3H]morphine entered both the brain and CSF after a 20-min brain perfusion, compared with [3H]CTAP. The increased CNS penetration observed for [3H]morphine, compared with [3H]CTAP, is likely due to the increased lipophilicity of morphine, as shown by its higher octanol/saline partition coefficient. Based on the pharmacokinetic profile, CTAP may be a promising mu-selective antagonist that can be used as a treatment for opiate overdose or addiction and also as a pharmacological tool to further understand opioid neurobiology.
Novel peptidic mu opioid antagonists: pharmacologic characterization in vitro and in vivo.[Pubmed:2566679]
J Pharmacol Exp Ther. 1989 May;249(2):544-51.
A series of six synthetic oCTAPeptides, structurally related to somatostatin, demonstrate high affinity and selectivity for mu opioid receptors in radioligand binding assays. The compounds, D-Phe-Cys-Tyr-D-Trp-Lys-Thr-Pen-Thr-NH2 (CTP), D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP), D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP), D-tetrahydroisoquinoline carboxylic acid (D-Tic)-Cys-Tyr-D-Trp-Lys-Thr-Pen-Thr-NH2 (D-Tic-CTP), D-Tic-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (D-Tic-CTOP) and D-Tic-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (D-Tic-CTAP), were tested in vitro and in vivo for agonist and antagonist potency and selectivity. In vitro bioassays included the guinea pig ileum, mouse vas deferens and rabbit vas deferens. In vivo tests included hotplate antinociception and gastrointestinal transit inhibition, performed in mice. In vitro, all six derivatives were competitive, highly selective mu antagonists (pA2 values from 6.4-7.9). The compounds demonstrated varying degrees of intrinsic agonist activity especially in the mouse vas deferens, the least active being CTAP and D-Tic-CTAP, which showed no mu or kappa agonist actions, and delta activity only at very high (greater than 3 microM) concentrations. In vivo, none of these compounds showed antinociceptive actions when administered i.c.v. in mice. All were competitive mu antagonists in the hotplate antinociception test.(ABSTRACT TRUNCATED AT 250 WORDS)
Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for mu opioid receptors.[Pubmed:2878079]
J Med Chem. 1986 Nov;29(11):2370-5.
A series of cyclic, conformationally constrained peptides related to somatostatin were designed and synthesized in an effort to develop highly selective and potent peptides for the mu opioid receptor. The following new peptides were prepared and tested for their mu opioid receptor potency and selectively in rat brain binding assays: D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (2, CTOP); D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (3, CTAP); D-Phe-Cys-Tyr-D-Trp-Nle-Thr-Pen-Thr-NH2 (4); D-Phe-Cys-Tyr-D-Trp-Lys-Val-Pen-Thr-NH2 (5); D-Phe-Cys-Tyr-D-Trp-Lys-Gly-Pen-Thr-NH2 (6); D-Phe-Cys-Tyr-Trp-Lys-Thr-Pen-Thr-NH2 (7); D-Tyr-Cys-Tyr-D-Trp-Lys-Thr-Cys-Thr-OH (8); D-PhGly-Cys-Tyr-D-Trp-Lys-Thr-Pen-Thr-NH2 (9); and D-PhGly-Pen-Phe-D-Trp-Lys-Thr-Cys-Thr-OH (10). The most selective peptide, 2 (CTOP), displayed both high affinity (IC50 = 3.5 nM) and exceptional selectivity (IC50 delta/IC50 mu = 4,000) for mu opioid receptors. Furthermore, 2 exhibited very low affinity for somatostatin receptors in the rat brain (IC50 greater than 24,000 nM), with an IC50 somatostatin/IC50 mu receptor selectivity of 8,750. These conformationally constrained cyclic peptides should provide new insight into the structural and conformational requirements for the mu opioid receptor and the physiological role of this receptor.


