TelotristatTPH inhibitor CAS# 1033805-28-5 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Rosuvastatin Calcium
Catalog No.:BCC3853
CAS No.:147098-20-2
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1033805-28-5 | SDF | Download SDF |
| PubChem ID | 25025298 | Appearance | Powder |
| Formula | C25H22ClF3N6O3 | M.Wt | 546.93 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LP-778902 | ||
| Solubility | DMSO : 33.33 mg/mL (60.94 mM; Need ultrasonic) | ||
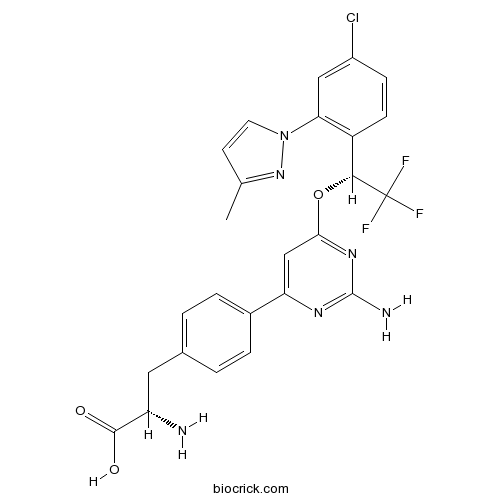
| Chemical Name | (2S)-2-amino-3-[4-[2-amino-6-[(1R)-1-[4-chloro-2-(3-methylpyrazol-1-yl)phenyl]-2,2,2-trifluoroethoxy]pyrimidin-4-yl]phenyl]propanoic acid | ||
| SMILES | CC1=NN(C=C1)C2=C(C=CC(=C2)Cl)C(C(F)(F)F)OC3=NC(=NC(=C3)C4=CC=C(C=C4)CC(C(=O)O)N)N | ||
| Standard InChIKey | NCLGDOBQAWBXRA-PGRDOPGGSA-N | ||
| Standard InChI | InChI=1S/C25H22ClF3N6O3/c1-13-8-9-35(34-13)20-11-16(26)6-7-17(20)22(25(27,28)29)38-21-12-19(32-24(31)33-21)15-4-2-14(3-5-15)10-18(30)23(36)37/h2-9,11-12,18,22H,10,30H2,1H3,(H,36,37)(H2,31,32,33)/t18-,22+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Telotristat (LP-778902) is a potent tryptophan hydroxylase inhibitor with an in vivo IC50 of 0.028 μM.In Vitro:Telotristat is the active moiety of telotristat etiprate. Telotristat etiprate is an ethyl ester prodrug which is hydrolyzed to telotristat. Telotristat etiprate is orally available serotonin synthesis inhibitor for the treatment of carcinoid syndrome[1].In Vivo:Telotristat etiprate is present in very low levels after oral administration. These low levels are due to rapid hydrolysis into the active moiety telotristat. The half-life ranges from approximately 4-12 h. There is no accumulation of telotristat with multiple dose administration over 2 weeks. Exposure to telotristat is approximately dose proportional[1]. References: | |||||

Telotristat Dilution Calculator

Telotristat Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8284 mL | 9.1419 mL | 18.2839 mL | 36.5678 mL | 45.7097 mL |
| 5 mM | 0.3657 mL | 1.8284 mL | 3.6568 mL | 7.3136 mL | 9.1419 mL |
| 10 mM | 0.1828 mL | 0.9142 mL | 1.8284 mL | 3.6568 mL | 4.571 mL |
| 50 mM | 0.0366 mL | 0.1828 mL | 0.3657 mL | 0.7314 mL | 0.9142 mL |
| 100 mM | 0.0183 mL | 0.0914 mL | 0.1828 mL | 0.3657 mL | 0.4571 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Telotristat is the active metabolite of LX1606(Telotristat etiprate), which is an orally bioavailable, small-molecule, tryptophan hydroxylase (TPH) inhibitor with potential antiserotonergic activity.
- Salidroside
Catalog No.:BCN5966
CAS No.:10338-51-9
- GNF-5837
Catalog No.:BCC3668
CAS No.:1033769-28-6
- Itol A
Catalog No.:BCN5847
CAS No.:1033747-78-2
- GNE-493
Catalog No.:BCC8048
CAS No.:1033735-94-2
- 1-Methyl-L-4,5-dihydroorotic acid
Catalog No.:BCC8472
CAS No.:103365-69-1
- L-364,373
Catalog No.:BCC7445
CAS No.:103342-82-1
- 3-Oxo-4-aza-5-alpha-androstane-17β-carboxylic acid
Catalog No.:BCC8641
CAS No.:103335-55-3
- Pre-schisanartanin B
Catalog No.:BCN5846
CAS No.:1033288-92-4
- Taltirelin
Catalog No.:BCC5271
CAS No.:103300-74-9
- LDK378
Catalog No.:BCC3691
CAS No.:1032900-25-6
- GSK1292263
Catalog No.:BCC3786
CAS No.:1032823-75-8
- PTIQ
Catalog No.:BCC7953
CAS No.:1032822-42-6
- HPGDS inhibitor 1
Catalog No.:BCC4065
CAS No.:1033836-12-2
- 1-O-Methylnataloe-emodin
Catalog No.:BCN7036
CAS No.:103392-51-4
- Octyl gallate
Catalog No.:BCN8432
CAS No.:1034-01-1
- Disodium (R)-2-Hydroxyglutarate
Catalog No.:BCC6515
CAS No.:103404-90-6
- Devazepide
Catalog No.:BCC7319
CAS No.:103420-77-5
- CTOP
Catalog No.:BCC5780
CAS No.:103429-31-8
- CTAP
Catalog No.:BCC5776
CAS No.:103429-32-9
- Z-Cyclopentyl-AP4
Catalog No.:BCC7616
CAS No.:103439-17-4
- Xanthone V1a
Catalog No.:BCN7922
CAS No.:103450-96-0
- NMS-1286937
Catalog No.:BCC5358
CAS No.:1034616-18-6
- Maprotiline HCl
Catalog No.:BCC4329
CAS No.:10347-81-6
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
Patient-reported Symptom Experiences in Patients With Carcinoid Syndrome After Participation in a Study of Telotristat Etiprate: A Qualitative Interview Approach.[Pubmed:27041406]
Clin Ther. 2016 Apr;38(4):759-68.
PURPOSE: Telotristat etiprate, a tryptophan hydroxylase inhibitor, was previously evaluated in a Phase II randomized, placebo-controlled clinical trial in patients with carcinoid syndrome (CS) and diarrhea not adequately controlled by octreotide. The objective of the current study was to characterize the symptom experiences of patients participating in that trial. METHODS: Consenting patients participated in one-on-one, qualitative interviews focused on eliciting symptoms they had experienced in association with their CS diagnosis and recollection of symptom changes they experienced while participating in the Phase II trial. FINDINGS: Among the 23 patients who participated in the previous 4-week dose-escalation study, 16 were eligible for interviews and 11 participated in the present study. The median time from study completion to the interview was 31 months; 4 of 11 patients were receiving Telotristat etiprate in a follow-up, open-label trial at the time of interview. All of the patients (100%) described diarrhea as a symptom of CS, with effects on the emotional, social, and physical aspects of their lives. Improvement in diarrhea during the study was described by 82% of participants, and was very impactful in several patients. Results led to the design and implementation of a larger interview program in Phase III and helped to establish a definition of clinically meaningful change for the clinical development program. IMPLICATIONS: The diarrhea associated with CS can have a large impact on daily lives, and patient interviews can characterize and capture clinically meaningful improvements with treatment. ClinicalTrials.gov Identifier: NCT00853047.
Telotristat Ethyl, a Tryptophan Hydroxylase Inhibitor for the Treatment of Carcinoid Syndrome.[Pubmed:27918724]
J Clin Oncol. 2017 Jan;35(1):14-23.
Purpose Preliminary studies suggested that Telotristat ethyl, a tryptophan hydroxylase inhibitor, reduces bowel movement (BM) frequency in patients with carcinoid syndrome. This placebo-controlled phase III study evaluated Telotristat ethyl in this setting. Patients and Methods Patients (N = 135) experiencing four or more BMs per day despite stable-dose somatostatin analog therapy received (1:1:1) placebo, Telotristat ethyl 250 mg, or Telotristat ethyl 500 mg three times per day orally during a 12-week double-blind treatment period. The primary end point was change from baseline in BM frequency. In an open-label extension, 115 patients subsequently received Telotristat ethyl 500 mg. Results Estimated differences in BM frequency per day versus placebo averaged over 12 weeks were -0.81 for Telotristat ethyl 250 mg ( P < .001) and 0.69 for Telotristat ethyl 500 mg ( P < .001). At week 12, mean BM frequency reductions per day for placebo, Telotristat ethyl 250 mg, and Telotristat ethyl 500 mg were -0.9, -1.7, and -2.1, respectively. Responses, predefined as a BM frequency reduction >/= 30% from baseline for >/= 50% of the double-blind treatment period, were observed in 20%, 44%, and 42% of patients given placebo, Telotristat ethyl 250 mg, and Telotristat ethyl 500 mg, respectively. Both Telotristat ethyl dosages significantly reduced mean urinary 5-hydroxyindole acetic acid versus placebo at week 12 ( P < .001). Mild nausea and asymptomatic increases in gamma-glutamyl transferase were observed in some patients receiving Telotristat ethyl. Follow-up of patients during the open-label extension revealed no new safety signals and suggested sustained BM responses to treatment. Conclusion Among patients with carcinoid syndrome not adequately controlled by somatostatin analogs, treatment with Telotristat ethyl was generally safe and well tolerated and resulted in significant reductions in BM frequency and urinary 5-hydroxyindole acetic acid.
Blocking peripheral serotonin synthesis by telotristat etiprate (LX1032/LX1606) reduces severity of both chemical- and infection-induced intestinal inflammation.[Pubmed:26206858]
Am J Physiol Gastrointest Liver Physiol. 2015 Sep 15;309(6):G455-65.
Mucosal inflammation is accompanied by an alteration in 5-HT. Intestinal 5-HT synthesis is catalyzed by tryptophan hydroxylase 1 (Tph1) and we have shown that mice deficient in this rate-limiting enzyme have reduced severity of intestinal inflammation in models of chemical-induced experimental colitis. Here, we investigated the effect of blocking peripheral 5-HT synthesis in generation of intestinal inflammation by a using peripheral Tph inhibitor, Telotristat etiprate (LX1606), in models of intestinal inflammation. LX1606 was given orally either prophylactically or therapeutically to mice with dextran sulfate sodium (DSS)-induced colitis or with infection with Trichuris muris. Severity of intestinal inflammation was measured by assessment of disease activity scores, histological damage, and MPO and inflammatory cytokine levels. LX1606 significantly reduced intestinal 5-HT levels and delayed onset and severity of DSS-induced acute and chronic colitis. This was associated with decreased MPO and proinflammatory cytokine levels compared with vehicle-treated controls. In the infection-induced inflammation model, treatment with LX1606 enhanced worm expulsion as well as increased IL-10 production and goblet cell numbers. LX1606-treated mice had significantly lower MPO and IL-1beta levels compared with controls postinfection. Our results demonstrate that peripheral 5-HT plays an important role in intestinal inflammation and in the generation of immune responses. Pharmacological reduction of peripheral 5-HT may serve as a potential strategy for modulating various intestinal inflammatory disorders.
Telotristat ethyl: a new option for the management of carcinoid syndrome.[Pubmed:27817224]
Expert Opin Pharmacother. 2016 Dec;17(18):2487-2498.
INTRODUCTION: Many patients with neuroendocrine tumour-related carcinoid syndrome treated with somatostatin analogues (SSA) won't achieve adequate symptom relief with the SSA alone; new treatment options are required. Telotristat ethyl is a tryptophan hydroxylase inhibitor, developed for the treatment of carcinoid syndrome. Areas covered: This review summarises the evidence supporting the role of Telotristat ethyl in the management of carcinoid syndrome. Rationale, pharmacodynamics, pharmacokinetics, metabolism, clinical experience, efficacy and toxicity profiles are covered. Expert opinion: The efficacy of Telotristat ethyl in producing a statistically-significant and clinically-meaningful reduction in daily bowel movements has been confirmed in phase III clinical trials. Two pivotal trials, TELESTAR and TELECAST, explored the role of Telotristat ethyl in the management of patients with carcinoid syndrome refractory to SSAs focusing on patients with >/=4 and <4 daily bowel movements, respectively. In addition, benefit was confirmed in patient-reported outcomes. Based on activity and safe toxicity profile, Telotristat ethyl is pending regulatory agencies evaluation and is likely to add to the armamentarium used to treat carcinoid syndrome. Long-term safety and efficacy data will be available from the ongoing TELEPATH study. The impact on carcinoid heart disease, mesenteric fibrosis and other long-term complications of carcinoid syndrome as well as its role earlier in patients' pathways remain investigational.


