GSK1292263Novel GPR119 agonist CAS# 1032823-75-8 |
- TAK-875
Catalog No.:BCC3702
CAS No.:1000413-72-8
- GPR40 Activator 1
Catalog No.:BCC4125
CAS No.:1309435-60-6
- TUG-770
Catalog No.:BCC2018
CAS No.:1402601-82-4
- GW-1100
Catalog No.:BCC1610
CAS No.:306974-70-9
- GW9508
Catalog No.:BCC1102
CAS No.:885101-89-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1032823-75-8 | SDF | Download SDF |
| PubChem ID | 24996872 | Appearance | Powder |
| Formula | C23H28N4O4S | M.Wt | 456.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 30 mg/mL (65.71 mM) *"≥" means soluble, but saturation unknown. | ||
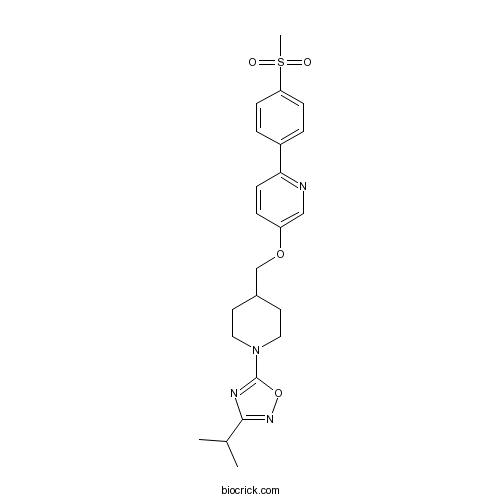
| Chemical Name | 5-[4-[[6-(4-methylsulfonylphenyl)pyridin-3-yl]oxymethyl]piperidin-1-yl]-3-propan-2-yl-1,2,4-oxadiazole | ||
| SMILES | CC(C)C1=NOC(=N1)N2CCC(CC2)COC3=CN=C(C=C3)C4=CC=C(C=C4)S(=O)(=O)C | ||
| Standard InChIKey | AYJRTVVIBJSSKN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H28N4O4S/c1-16(2)22-25-23(31-26-22)27-12-10-17(11-13-27)15-30-19-6-9-21(24-14-19)18-4-7-20(8-5-18)32(3,28)29/h4-9,14,16-17H,10-13,15H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GSK1292263 is a novel agonist of GPR119. | |||||
| Targets | GPR119 | |||||

GSK1292263 Dilution Calculator

GSK1292263 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1903 mL | 10.9515 mL | 21.9029 mL | 43.8059 mL | 54.7573 mL |
| 5 mM | 0.4381 mL | 2.1903 mL | 4.3806 mL | 8.7612 mL | 10.9515 mL |
| 10 mM | 0.219 mL | 1.0951 mL | 2.1903 mL | 4.3806 mL | 5.4757 mL |
| 50 mM | 0.0438 mL | 0.219 mL | 0.4381 mL | 0.8761 mL | 1.0951 mL |
| 100 mM | 0.0219 mL | 0.1095 mL | 0.219 mL | 0.4381 mL | 0.5476 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK1292263 is a novel agonist of GPR119 receptor agonist and is used for the treatment of type 2 diabetes.
GPR119 is described as a class A (rhodopsin-type) orphan GPCR with no close primary sequence relative in the human genome. The activation of GPR119 increases the intracellular accumulation of cAMP, resulting in enhanced insulin secretion from pancreatic β-cells and increased release of the gut peptides GLP-1 (glucagon-like peptide 1), GIP (glucose-dependent insulinotropic peptide) and PYY (polypeptide YY).
In vitro, GSK1292263 treatment displayed little inhibition towards CYPs (CYP1A2, 2C9, 2C19, 2D6, 3A4), p-GP, OATP1B3, or OCT2. However, GSK1292263 inhibited BCRP and OATP1B1, which are transporters involved in statin disposition 1.
In the glucose tolerance test in rats, administration of GSK-1292263 significantly increases the peak insulin response and insulin AUC (0-15 min) as compared with the values in the vehicle control. The upregulation of insulin was found to correlate with an increase in the glucose disposal rate. In hyperinsulinemic-euglycemic clamps, GSK-1292263 administration on Sprague-Dawley rats at dose of 10 or 30 mg/kg 2 hours prior to insulin infusion can promote glucagon secretion with no increase of blood glucose levels 2.
References:
1. Polli JW, Hussey E, Bush M, et al. Evaluation of drug interactions of GSK1292263 (a GPR119 agonist) with statins: from in vitro data to clinical study design. Xenobiotica; the fate of foreign compounds in biological systems. 2013;43(6):498-508.
2. Zhu X, Huang D, Lan X, et al. The first pharmacophore model for potent G protein-coupled receptor 119 agonist. European journal of medicinal chemistry. 2011;46(7):2901-2907.
- PTIQ
Catalog No.:BCC7953
CAS No.:1032822-42-6
- GDC-0980 (RG7422)
Catalog No.:BCC4992
CAS No.:1032754-93-0
- GNE-477
Catalog No.:BCC8049
CAS No.:1032754-81-6
- BAY 80-6946 (Copanlisib)
Catalog No.:BCC4986
CAS No.:1032568-63-0
- L-655,240
Catalog No.:BCC7156
CAS No.:103253-15-2
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- D-Arabinose
Catalog No.:BCN3791
CAS No.:10323-20-3
- A939572
Catalog No.:BCC5305
CAS No.:1032229-33-6
- Fmoc-Cys(Trt)-OH
Catalog No.:BCC3479
CAS No.:103213-32-7
- Fmoc-Tyr(3,5-I2)-OH
Catalog No.:BCC3264
CAS No.:103213-31-6
- Pranlukast
Catalog No.:BCC4827
CAS No.:103177-37-3
- 14-Norpseurotin A
Catalog No.:BCN7262
CAS No.:1031727-34-0
- LDK378
Catalog No.:BCC3691
CAS No.:1032900-25-6
- Taltirelin
Catalog No.:BCC5271
CAS No.:103300-74-9
- Pre-schisanartanin B
Catalog No.:BCN5846
CAS No.:1033288-92-4
- 3-Oxo-4-aza-5-alpha-androstane-17β-carboxylic acid
Catalog No.:BCC8641
CAS No.:103335-55-3
- L-364,373
Catalog No.:BCC7445
CAS No.:103342-82-1
- 1-Methyl-L-4,5-dihydroorotic acid
Catalog No.:BCC8472
CAS No.:103365-69-1
- GNE-493
Catalog No.:BCC8048
CAS No.:1033735-94-2
- Itol A
Catalog No.:BCN5847
CAS No.:1033747-78-2
- GNF-5837
Catalog No.:BCC3668
CAS No.:1033769-28-6
- Salidroside
Catalog No.:BCN5966
CAS No.:10338-51-9
- Telotristat
Catalog No.:BCC5128
CAS No.:1033805-28-5
- HPGDS inhibitor 1
Catalog No.:BCC4065
CAS No.:1033836-12-2
Gut hormone pharmacology of a novel GPR119 agonist (GSK1292263), metformin, and sitagliptin in type 2 diabetes mellitus: results from two randomized studies.[Pubmed:24699248]
PLoS One. 2014 Apr 3;9(4):e92494.
UNLABELLED: GPR119 receptor agonists improve glucose metabolism and alter gut hormone profiles in animal models and healthy subjects. We therefore investigated the pharmacology of GSK1292263 (GSK263), a selective GPR119 agonist, in two randomized, placebo-controlled studies that enrolled subjects with type 2 diabetes. Study 1 had drug-naive subjects or subjects who had stopped their diabetic medications, and Study 2 had subjects taking metformin. GSK263 was administered as single (25-800 mg; n = 45) or multiple doses (100-600 mg/day for 14 days; n = 96). Placebo and sitagliptin 100 mg/day were administered as comparators. In Study 1, sitagliptin was co-administered with GSK263 or placebo on Day 14 of dosing. Oral glucose and meal challenges were used to assess the effects on plasma glucose, insulin, C-peptide, glucagon, peptide tyrosine-tyrosine (PYY), glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). After 13 days of dosing, GSK263 significantly increased plasma total PYY levels by approximately five-fold compared with placebo, reaching peak concentrations of approximately 50 pM after each of the three standardized meals with the 300 mg BID dose. Co-dosing of GSK263 and metformin augmented peak concentrations to approximately 100 pM at lunchtime. GSK263 had no effect on active or total GLP-1 or GIP, but co-dosing with metformin increased post-prandial total GLP-1, with little effect on active GLP-1. Sitagliptin increased active GLP-1, but caused a profound suppression of total PYY, GLP-1, and GIP when dosed alone or with GSK263. This suppression of peptides was reduced when sitagliptin was co-dosed with metformin. GSK263 had no significant effect on circulating glucose, insulin, C-peptide or glucagon levels. We conclude that GSK263 did not improve glucose control in type 2 diabetics, but it had profound effects on circulating PYY. The gut hormone effects of this GPR119 agonist were modulated when co-dosed with metformin and sitagliptin. Metformin may modulate negative feedback loops controlling the secretion of enteroendocrine peptides. TRIAL REGISTRATION: Clinicaltrials.gov NCT01119846 Clinicaltrials.gov NCT01128621.
Evaluation of drug interactions of GSK1292263 (a GPR119 agonist) with statins: from in vitro data to clinical study design.[Pubmed:23256625]
Xenobiotica. 2013 Jun;43(6):498-508.
1. This work investigated the drug interaction potential of GSK1292263, a novel GPR119 agonist, with the HMG-coA reductase inhibitors simvastatin and rosuvastatin. 2. In vitro experiments assessed the inhibition of transporters and CYP enzymes by GSK1292263, and a clinical drug interaction study investigated the effect of GSK1292263 (300 mg BID) on the pharmacokinetic profile of simvastatin (40 mg single dose) and rosuvastatin (10 mg single dose). 3. In vitro, GSK1292263 demonstrated little/weak inhibition (IC50 values >30 muM) towards CYPs (CYP1A2, 2C9, 2C19, 2D6, 3A4), Pgp, OATP1B3, or OCT2. However, GSK1292263 inhibited BCRP and OATP1B1, which are transporters involved in statin disposition. 4. In the clinical study, small increases in the AUC(0-inf) of simvastatin [mean ratio (90% CI) of 1.34 (1.22, 1.48)] and rosuvastatin [mean ratio (90% CI) of 1.39 (1.30, 1.49)] were observed when co-administered with GSK1292263, which is consistent with an inhibitory effect on intestinal BCRP and CYP3A4. In contrast, GSK1292263 did not inhibit OATP1B1 based on the lack of changes in simvastatin acid exposure [mean AUC(0-inf) ratio (90% CI) of 1.05 (0.91, 1.21)]. 5. GSK1292263 has a weak drug interaction with simvastatin and rosuvastain. This study provides a mechanistic understanding of the in vivo inhibition of transporters and enzymes by GSK1292263.


