TAK-875GPR40 agonist CAS# 1000413-72-8 |
- TUG-770
Catalog No.:BCC2018
CAS No.:1402601-82-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1000413-72-8 | SDF | Download SDF |
| PubChem ID | 24857286 | Appearance | Powder |
| Formula | C29H32O7S | M.Wt | 524.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Fasiglifam | ||
| Solubility | DMSO : ≥ 128 mg/mL (243.98 mM) *"≥" means soluble, but saturation unknown. | ||
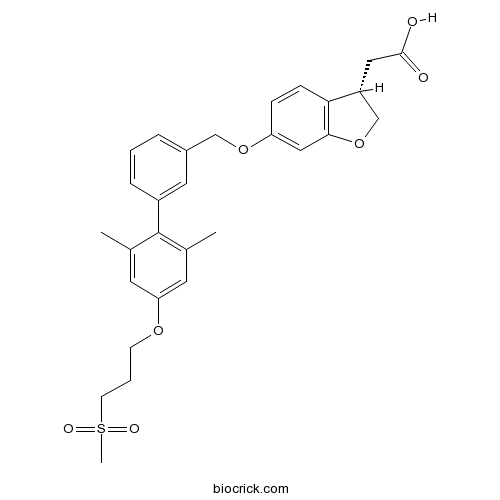
| Chemical Name | 2-[(3S)-6-[[3-[2,6-dimethyl-4-(3-methylsulfonylpropoxy)phenyl]phenyl]methoxy]-2,3-dihydro-1-benzofuran-3-yl]acetic acid | ||
| SMILES | CC1=CC(=CC(=C1C2=CC(=CC=C2)COC3=CC4=C(C=C3)C(CO4)CC(=O)O)C)OCCCS(=O)(=O)C | ||
| Standard InChIKey | BZCALJIHZVNMGJ-HSZRJFAPSA-N | ||
| Standard InChI | InChI=1S/C29H32O7S/c1-19-12-25(34-10-5-11-37(3,32)33)13-20(2)29(19)22-7-4-6-21(14-22)17-35-24-8-9-26-23(15-28(30)31)18-36-27(26)16-24/h4,6-9,12-14,16,23H,5,10-11,15,17-18H2,1-3H3,(H,30,31)/t23-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | TAK-875 is a selective agonist of GPR40 with EC50 of 14 nM, 400-fold more potent than oleic acid. | |||||
| Targets | GPR40 | |||||
| IC50 | 14 nM (EC50) | |||||

TAK-875 Dilution Calculator

TAK-875 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9061 mL | 9.5303 mL | 19.0607 mL | 38.1214 mL | 47.6517 mL |
| 5 mM | 0.3812 mL | 1.9061 mL | 3.8121 mL | 7.6243 mL | 9.5303 mL |
| 10 mM | 0.1906 mL | 0.953 mL | 1.9061 mL | 3.8121 mL | 4.7652 mL |
| 50 mM | 0.0381 mL | 0.1906 mL | 0.3812 mL | 0.7624 mL | 0.953 mL |
| 100 mM | 0.0191 mL | 0.0953 mL | 0.1906 mL | 0.3812 mL | 0.4765 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
TAK-875 is a potent, selective, and oral GPR40 agonist. GPR40 is one of the G protein-coupled receptors predominantly expressed in pancreatic β-cells, mediating enhancement of glucose-stimulated insulin secretion by free fatty acids.
In vitro: TAK-875 exhibited potent agonist activity and high binding affinity to the human receptor. In addition, TAK-875 showed excellent agonist potency selectivity for GPR40 receptor over other members of the FFA receptor family (for which EC50>10 μM) [1].
In vivo: TAK-875 showed potent plasma glucose-lowering action and insulinotropic action during an oral glucose tolerance test in female Wistar fatty rats with impaired glucose tolerance [2].
Clinical trial: TAK-875 acts as a glucose-dependent insulinotropic agent with low hypoglycemic risk. Its P K is suitable for once-daily oral administration.
References:
[1] Negoro N, Sasaki S, Mikami S, Ito M, Suzuki M, Tsujihata Y, Ito R, Harada A, Takeuchi K, Suzuki N, Miyazaki J, Santou T, Odani T, Kanzaki N, Funami M, Tanaka T1, Kogame A, Matsunaga S, Yasuma T, Momose Y. Discovery of TAK-875: A Potent, Selective, and Orally Bioavailable GPR40 Agonist. ACS Med Chem Lett. 2010 Jun 18;1(6):290-4.
[2] Leifke E, Naik H, Wu J, Viswanathan P, Demanno D, Kipnes M, Vakilynejad M. A multiple-ascending-dose study to evaluate safety, pharmacokinetics, and pharmacodynamics of a novel GPR40 agonist, TAK-875, in subjects with type 2 diabetes. Clin Pharmacol Ther. 2012 Jul;92(1):29-39.
- GIP (human)
Catalog No.:BCC5870
CAS No.:100040-31-1
- A-867744
Catalog No.:BCC1324
CAS No.:1000279-69-5
- 2-Methylthioadenosine triphosphate tetrasodium salt
Catalog No.:BCC6918
CAS No.:100020-57-3
- Dauriporphinoline
Catalog No.:BCN7901
CAS No.:100009-82-3
- Anisole
Catalog No.:BCN2619
CAS No.:100-66-3
- Benzaldehyde
Catalog No.:BCN8529
CAS No.:100-52-7
- Benzylamine
Catalog No.:BCN1789
CAS No.:100-46-9
- Pentamidine
Catalog No.:BCC3836
CAS No.:100-33-4
- 4-Methoxybenzoic acid
Catalog No.:BCN3838
CAS No.:100-09-4
- 8-Hydroxy-3,5,6,7,3',4'-hexamethoxyflavone
Catalog No.:BCN7870
CAS No.:1000415-56-4
- AS 19
Catalog No.:BCC7218
CAS No.:1000578-26-6
- KW 2449
Catalog No.:BCC2179
CAS No.:1000669-72-6
- Stilbostemin N
Catalog No.:BCN4741
CAS No.:1000676-45-8
- Tegobuvir
Catalog No.:BCC1991
CAS No.:1000787-75-6
- 7-Hydroxy-2',5,8-trimethoxyflavanone
Catalog No.:BCN5817
CAS No.:100079-34-3
- 2',4'-Dihydroxy-2,3',6'-trimethoxychalcone
Catalog No.:BCN1643
CAS No.:100079-39-8
- H-Lys(Tfa)-OH
Catalog No.:BCC2985
CAS No.:10009-20-8
- Chloramultilide B
Catalog No.:BCN6613
CAS No.:1000995-47-0
- Chloramultilide C
Catalog No.:BCN6618
CAS No.:1000995-48-1
- Chloramultilide D
Catalog No.:BCN7102
CAS No.:1000995-49-2
- GnRH Associated Peptide (GAP) (1-13), human
Catalog No.:BCC1013
CAS No.:100111-07-7
Long-term safety and efficacy of fasiglifam (TAK-875), a G-protein-coupled receptor 40 agonist, as monotherapy and combination therapy in Japanese patients with type 2 diabetes: a 52-week open-label phase III study.[Pubmed:27178047]
Diabetes Obes Metab. 2016 Sep;18(9):925-9.
This multicentre, open-label, phase III study investigated the safety and efficacy of the G-protein-coupled receptor 40 agonist fasiglifam. Japanese patients with type 2 diabetes and inadequate glycaemic control despite diet and/or exercise (n = 282), or despite diet and/or exercise plus one oral antidiabetic agent [sulphonylurea (n = 262), rapid-acting insulin secretagogue (n = 124), alpha-glucosidase inhibitor (n = 141), biguanide (n = 136), thiazolidinedione (n = 139) or dipeptidyl peptidase-4 inhibitor (n = 138)] were randomized to treatment with fasiglifam 25 or 50 mg once daily for 52 weeks. The primary endpoints were safety variables. The overall incidence of treatment-emergent adverse events (TEAEs) was 75.4-85.1% in the 25 mg group and 78.9-89.9% in the 50 mg group; most TEAEs were mild. Hypoglycaemia was negligible with fasiglifam monotherapy and most common with sulphonylurea combination therapy (12.4 and 9.1% for 25 and 50 mg groups, respectively). Abnormal liver-related laboratory values were uncommon. Glycated haemoglobin levels decreased from week 2 in all groups and were maintained to week 52. Although fasiglifam as monotherapy or in combination regimens was well tolerated during long-term treatment, global concerns about liver safety led to termination of its development after study completion.
Fasiglifam (TAK-875) Alters Bile Acid Homeostasis in Rats and Dogs: A Potential Cause of Drug Induced Liver Injury.[Pubmed:28108665]
Toxicol Sci. 2017 May 1;157(1):50-61.
Fasiglifam (TAK-875), a Free Fatty Acid Receptor 1 (FFAR1) agonist in development for the treatment of type 2 diabetes, was voluntarily terminated in phase 3 due to adverse liver effects. A mechanistic investigation described in this manuscript focused on the inhibition of bile acid (BA) transporters as a driver of the liver findings. TAK-875 was an in vitro inhibitor of multiple influx (NTCP and OATPs) and efflux (BSEP and MRPs) hepatobiliary BA transporters at micromolar concentrations. Repeat dose studies determined that TAK-875 caused a dose-dependent increase in serum total BA in rats and dogs. Additionally, there were dose-dependent increases in both unconjugated and conjugated individual BAs in both species. Rats had an increase in serum markers of liver injury without correlative microscopic signs of tissue damage. Two of 6 dogs that received the highest dose of TAK-875 developed liver injury with clinical pathology changes, and by microscopic analysis had portal granulomatous inflammation with neutrophils around a crystalline deposition. The BA composition of dog bile also significantly changed in a dose-dependent manner following TAK-875 administration. At the highest dose, levels of taurocholic acid were 50% greater than in controls with a corresponding 50% decrease in taurochenodeoxycholic acid. Transporter inhibition by TAK-875 may cause liver injury in dogs through altered bile BA composition characteristics, as evidenced by crystalline deposition, likely composed of test article, in the bile duct. In conclusion, a combination of in vitro and in vivo evidence suggests that BA transporter inhibition could contribute to TAK-875-mediated liver injury in dogs.
Fasiglifam (TAK-875) has dual potentiating mechanisms via Galphaq-GPR40/FFAR1 signaling branches on glucose-dependent insulin secretion.[Pubmed:27433346]
Pharmacol Res Perspect. 2016 Apr 27;4(3):e00237.
Fasiglifam (TAK-875) is a free fatty acid receptor 1 (FFAR1)/G-protein-coupled receptor 40 (GPR40) agonist that improves glycemic control in type 2 diabetes with minimum risk of hypoglycemia. Fasiglifam potentiates glucose-stimulated insulin secretion (GSIS) from pancreatic beta-cells glucose dependently, although the precise mechanism underlying the glucose dependency still remains unknown. Here, we investigated key cross-talk between the GSIS pathway and FFAR1 signaling, and Ca(2+) dynamics using mouse insulinoma MIN6 cells. We demonstrated that the glucose-dependent insulinotropic effect of fasiglifam required membrane depolarization and that fasiglifam induced a glucose-dependent increase in intracellular Ca(2+) level and amplification of Ca(2+) oscillations. This differed from the sulfonylurea glimepiride that induced changes in Ca(2+) dynamics glucose independently. Stimulation with cell-permeable analogs of IP3 or diacylglycerol (DAG), downstream second messengers of Galphaq-FFAR1, augmented GSIS similar to fasiglifam, indicating their individual roles in the potentiation of GSIS pathway. Intriguingly, the IP3 analog triggered similar Ca(2+) dynamics to fasiglifam, whereas the DAG analog had no effect. Despite the lack of an effect on Ca(2+) dynamics, the DAG analog elicited synergistic effects on insulin secretion with Ca(2+) influx evoked by an L-type voltage-dependent calcium channel opener that mimics glucose-dependent Ca(2+) dynamics. These results indicate that the Galphaq signaling activated by fasiglifam enhances GSIS pathway via dual potentiating mechanisms in which IP3 amplifies glucose-induced Ca(2+) oscillations and DAG/protein kinase C (PKC) augments downstream secretory mechanisms independent of Ca(2+) oscillations.
Fasiglifam (TAK-875): Mechanistic Investigation and Retrospective Identification of Hazards for Drug Induced Liver Injury.[Pubmed:28206647]
Toxicol Sci. 2018 Jun 1;163(2):374-384.
TAK-875, a GPR40 agonist, was withdrawn from Phase III clinical trials due to drug-induced liver injury (DILI). Mechanistic studies were conducted to identify potential DILI hazards (covalent binding burden (CVB), hepatic transporter inhibition, mitochondrial toxicity, and liver toxicity in rats) associated with TAK-875. Treatment of hepatocytes with radiolabeled TAK-875 resulted in a CVB of 2.0 mg/day, which is above the threshold of 1 mg/day considered to be a risk for DILI. Covalent binding to hepatocytes was due to formation of a reactive acyl glucuronide (AG) and, possibly, an acyl-CoA thioester intermediate. Formation of TAK-875AG in hepatocytes and/or in vivo was in the order of non-rodents > human (in vitro only) > rat. These data suggest that non-rodents, and presumably humans, form TAK-875AG more efficiently than rats, and that AG-mediated toxicities in rats may only occur at high doses. TAK-875 (1000 mg/kg/day) formed significant amounts of AG metabolite (TAK-875 and TAK-875AG had similar potencies (within 3-fold) for human multi-drug resistant associated protein 2/4 (MRP2/4) and bile salt export pump, but TAK-875AG was exceptionally potent against MRP3 (0.21 muM). Inhibition of MRPs may contribute to liver accumulation of TAK-875AG. TAK-875 also inhibited mitochondrial respiration in HepG2 cells, and mitochondrial Complex 1 and 2 activities in isolated rat mitochondria. In summary, formation of TAK-875AG, and possibly TAK-875CoA in hepatocytes, coupled with inhibition of hepatic transporters and mitochondrial respiration may be key contributors to TAK-875-mediated DILI.


